 Found 187 hits with Last Name = 'fushimi' and Initial = 'm'
Found 187 hits with Last Name = 'fushimi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524981
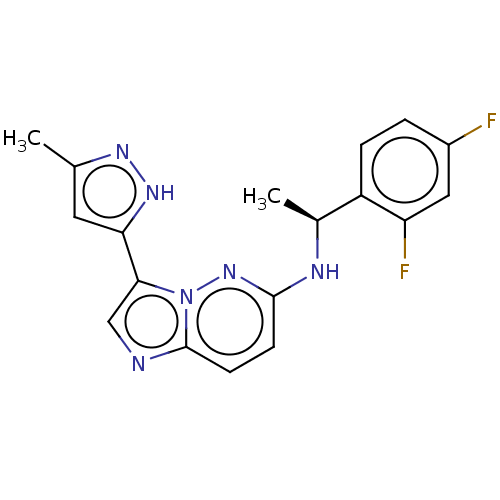
(CHEMBL4562879)Show SMILES C[C@H](Nc1ccc2ncc(-c3cc(C)n[nH]3)n2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C18H16F2N6/c1-10-7-15(24-23-10)16-9-21-18-6-5-17(25-26(16)18)22-11(2)13-4-3-12(19)8-14(13)20/h3-9,11H,1-2H3,(H,22,25)(H,23,24)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459632
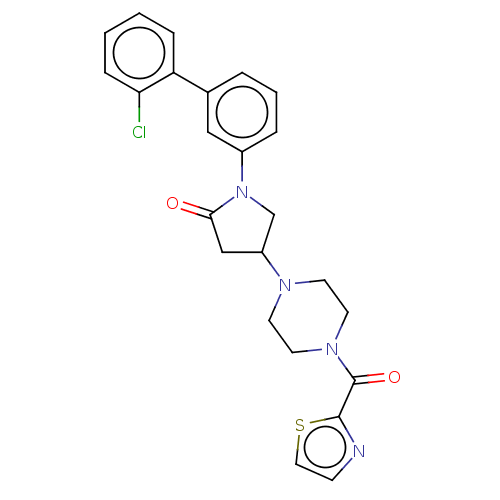
(CHEMBL4204645)Show SMILES Clc1ccccc1-c1cccc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C24H23ClN4O2S/c25-21-7-2-1-6-20(21)17-4-3-5-18(14-17)29-16-19(15-22(29)30)27-9-11-28(12-10-27)24(31)23-26-8-13-32-23/h1-8,13-14,19H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50180268
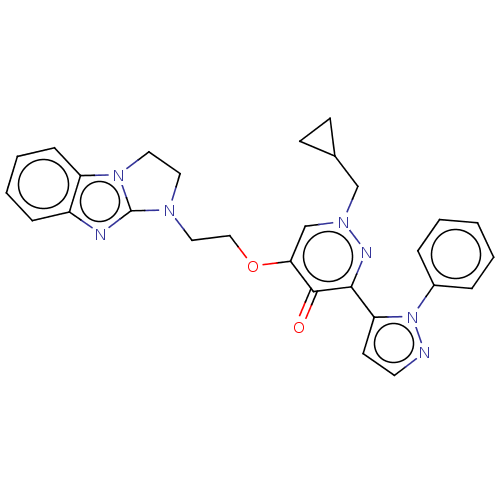
(CHEMBL3814662)Show SMILES O=c1c(OCCN2CCn3c2nc2ccccc32)cn(CC2CC2)nc1-c1ccnn1-c1ccccc1 Show InChI InChI=1S/C28H27N7O2/c36-27-25(37-17-16-32-14-15-34-23-9-5-4-8-22(23)30-28(32)34)19-33(18-20-10-11-20)31-26(27)24-12-13-29-35(24)21-6-2-1-3-7-21/h1-9,12-13,19-20H,10-11,14-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... |
Bioorg Med Chem 24: 3447-55 (2016)
Article DOI: 10.1016/j.bmc.2016.05.049
BindingDB Entry DOI: 10.7270/Q2H9974G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50180271
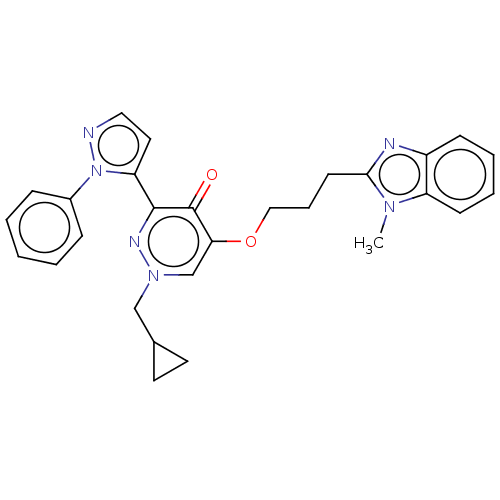
(CHEMBL3814601)Show SMILES Cn1c(CCCOc2cn(CC3CC3)nc(-c3ccnn3-c3ccccc3)c2=O)nc2ccccc12 Show InChI InChI=1S/C28H28N6O2/c1-32-23-11-6-5-10-22(23)30-26(32)12-7-17-36-25-19-33(18-20-13-14-20)31-27(28(25)35)24-15-16-29-34(24)21-8-3-2-4-9-21/h2-6,8-11,15-16,19-20H,7,12-14,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... |
Bioorg Med Chem 24: 3447-55 (2016)
Article DOI: 10.1016/j.bmc.2016.05.049
BindingDB Entry DOI: 10.7270/Q2H9974G |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607682
(CHEMBL5220685)Show SMILES COC(=O)[C@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459647
(CHEMBL4205102)Show SMILES Cc1ccccc1-c1cccc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C25H26N4O2S/c1-18-5-2-3-8-22(18)19-6-4-7-20(15-19)29-17-21(16-23(29)30)27-10-12-28(13-11-27)25(31)24-26-9-14-32-24/h2-9,14-15,21H,10-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524985
(CHEMBL4460367)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)S(C)(=O)=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C20H19F2N5O2S/c1-11-8-19(26-25-11)27-10-17(30(3,28)29)15-6-7-18(24-20(15)27)23-12(2)14-5-4-13(21)9-16(14)22/h4-10,12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524987

(CHEMBL4516801)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N(C)C)c1ccc(F)cn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-12-9-19(27-26-12)29-11-16(21(30)28(3)4)15-6-8-18(25-20(15)29)24-13(2)17-7-5-14(22)10-23-17/h5-11,13H,1-4H3,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607676
(CHEMBL5218698)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524983
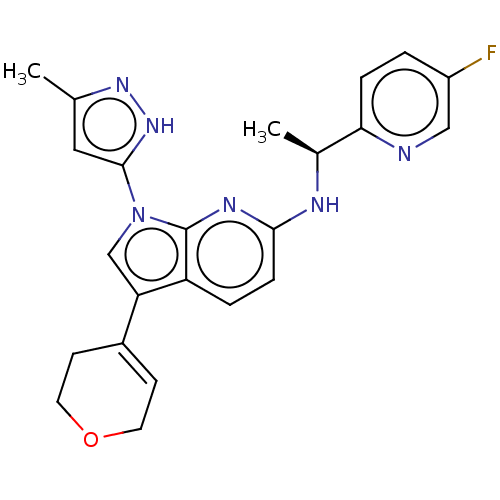
(CHEMBL4458269)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C1=CCOCC1)c1ccc(F)cn1 |r,t:21| Show InChI InChI=1S/C23H23FN6O/c1-14-11-22(29-28-14)30-13-19(16-7-9-31-10-8-16)18-4-6-21(27-23(18)30)26-15(2)20-5-3-17(24)12-25-20/h3-7,11-13,15H,8-10H2,1-2H3,(H,26,27)(H,28,29)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524981

(CHEMBL4562879)Show SMILES C[C@H](Nc1ccc2ncc(-c3cc(C)n[nH]3)n2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C18H16F2N6/c1-10-7-15(24-23-10)16-9-21-18-6-5-17(25-26(16)18)22-11(2)13-4-3-12(19)8-14(13)20/h3-9,11H,1-2H3,(H,22,25)(H,23,24)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607678

(CHEMBL5218878)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCNC(=O)C1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607681
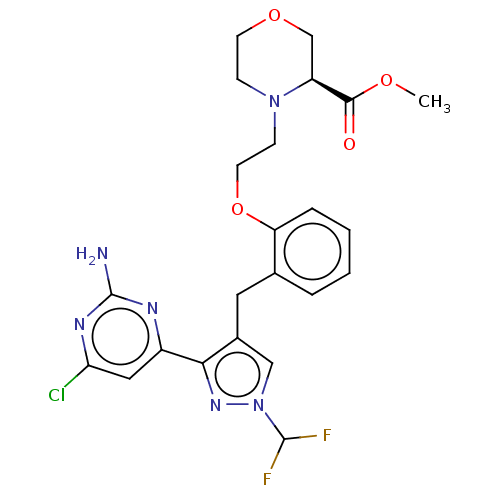
(CHEMBL5218552)Show SMILES COC(=O)[C@@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459633
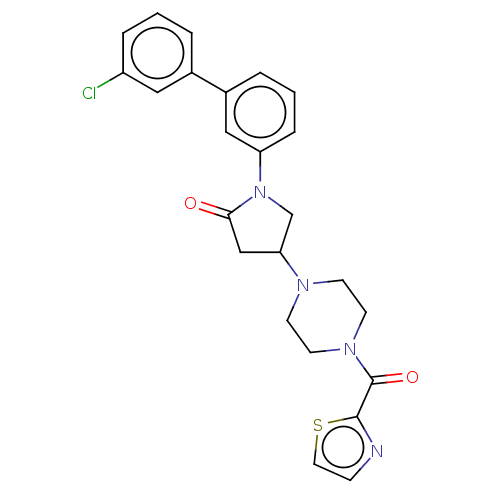
(CHEMBL4205134)Show SMILES Clc1cccc(c1)-c1cccc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C24H23ClN4O2S/c25-19-5-1-3-17(13-19)18-4-2-6-20(14-18)29-16-21(15-22(29)30)27-8-10-28(11-9-27)24(31)23-26-7-12-32-23/h1-7,12-14,21H,8-11,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607679
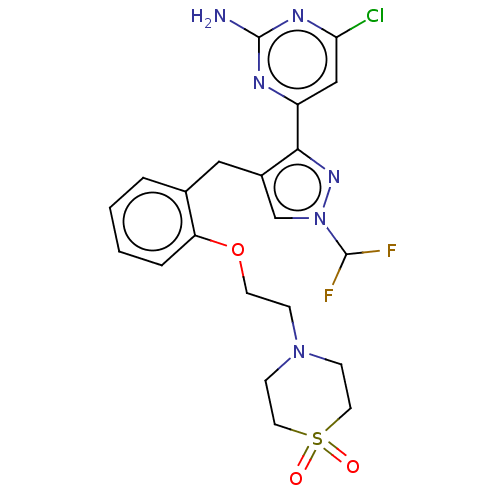
(CHEMBL5218615)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCS(=O)(=O)CC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524980
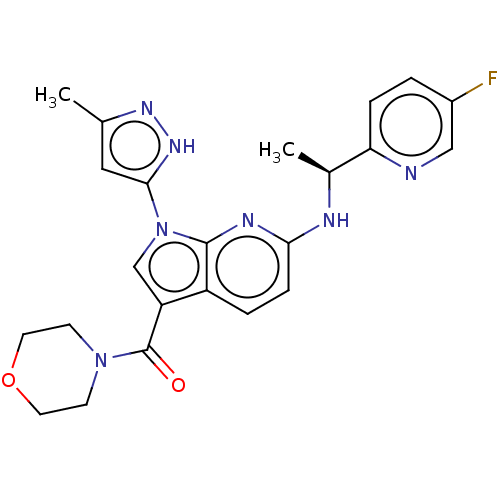
(CHEMBL4437605)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N1CCOCC1)c1ccc(F)cn1 |r| Show InChI InChI=1S/C23H24FN7O2/c1-14-11-21(29-28-14)31-13-18(23(32)30-7-9-33-10-8-30)17-4-6-20(27-22(17)31)26-15(2)19-5-3-16(24)12-25-19/h3-6,11-13,15H,7-10H2,1-2H3,(H,26,27)(H,28,29)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607682

(CHEMBL5220685)Show SMILES COC(=O)[C@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524983

(CHEMBL4458269)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C1=CCOCC1)c1ccc(F)cn1 |r,t:21| Show InChI InChI=1S/C23H23FN6O/c1-14-11-22(29-28-14)30-13-19(16-7-9-31-10-8-16)18-4-6-21(27-23(18)30)26-15(2)20-5-3-17(24)12-25-20/h3-7,11-13,15H,8-10H2,1-2H3,(H,26,27)(H,28,29)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524979
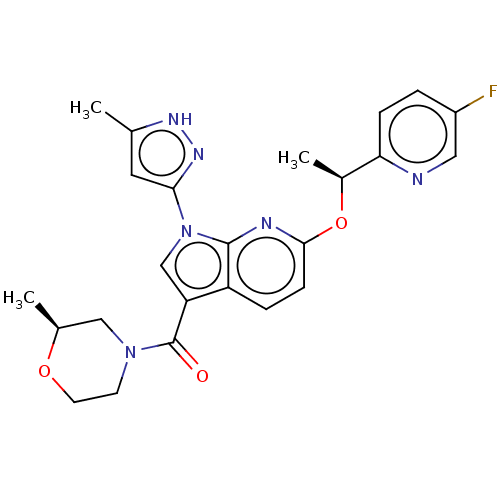
(CHEMBL4440381)Show SMILES C[C@H](Oc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)C(=O)N1CCO[C@@H](C)C1)c1ccc(F)cn1 |r| Show InChI InChI=1S/C24H25FN6O3/c1-14-10-21(29-28-14)31-13-19(24(32)30-8-9-33-15(2)12-30)18-5-7-22(27-23(18)31)34-16(3)20-6-4-17(25)11-26-20/h4-7,10-11,13,15-16H,8-9,12H2,1-3H3,(H,28,29)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524984
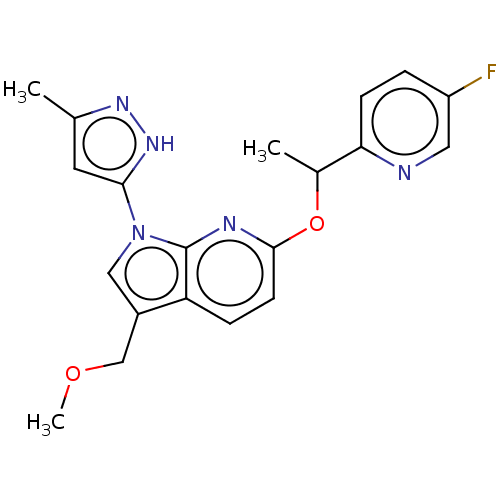
(CHEMBL4434659)Show SMILES COCc1cn(-c2cc(C)n[nH]2)c2nc(OC(C)c3ccc(F)cn3)ccc12 Show InChI InChI=1S/C20H20FN5O2/c1-12-8-18(25-24-12)26-10-14(11-27-3)16-5-7-19(23-20(16)26)28-13(2)17-6-4-15(21)9-22-17/h4-10,13H,11H2,1-3H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524984

(CHEMBL4434659)Show SMILES COCc1cn(-c2cc(C)n[nH]2)c2nc(OC(C)c3ccc(F)cn3)ccc12 Show InChI InChI=1S/C20H20FN5O2/c1-12-8-18(25-24-12)26-10-14(11-27-3)16-5-7-19(23-20(16)26)28-13(2)17-6-4-15(21)9-22-17/h4-10,13H,11H2,1-3H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524989
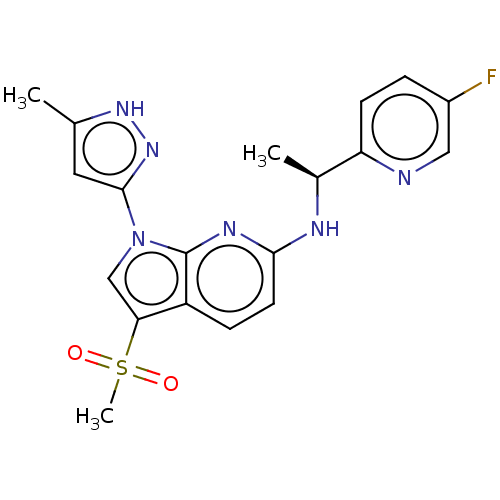
(CHEMBL4535072)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)S(C)(=O)=O)c1ccc(F)cn1 |r| Show InChI InChI=1S/C19H19FN6O2S/c1-11-8-18(25-24-11)26-10-16(29(3,27)28)14-5-7-17(23-19(14)26)22-12(2)15-6-4-13(20)9-21-15/h4-10,12H,1-3H3,(H,22,23)(H,24,25)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607683
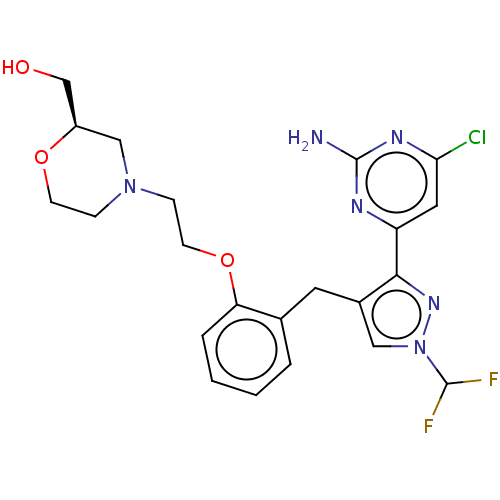
(CHEMBL5219579)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607686
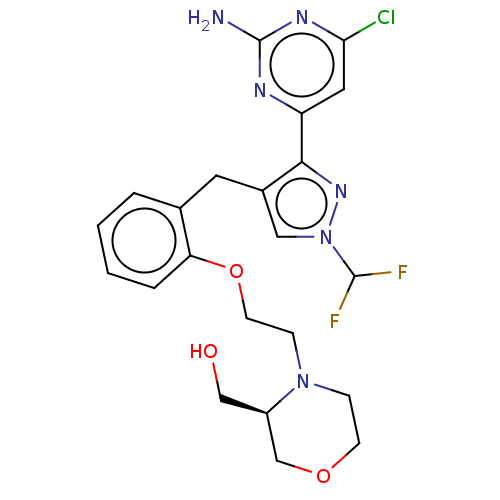
(CHEMBL5219830)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459645
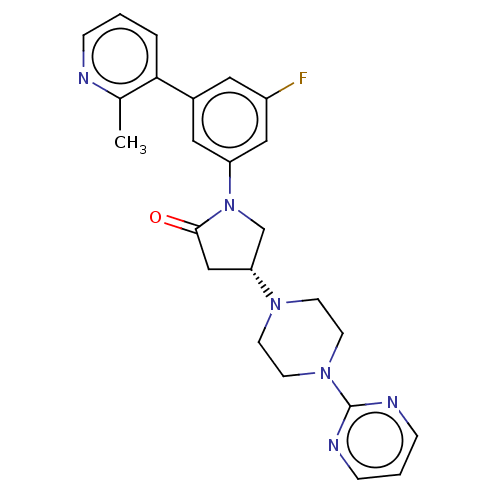
(CHEMBL4213030)Show SMILES Cc1ncccc1-c1cc(F)cc(c1)N1C[C@@H](CC1=O)N1CCN(CC1)c1ncccn1 |r| Show InChI InChI=1S/C24H25FN6O/c1-17-22(4-2-5-26-17)18-12-19(25)14-20(13-18)31-16-21(15-23(31)32)29-8-10-30(11-9-29)24-27-6-3-7-28-24/h2-7,12-14,21H,8-11,15-16H2,1H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607676

(CHEMBL5218698)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607680
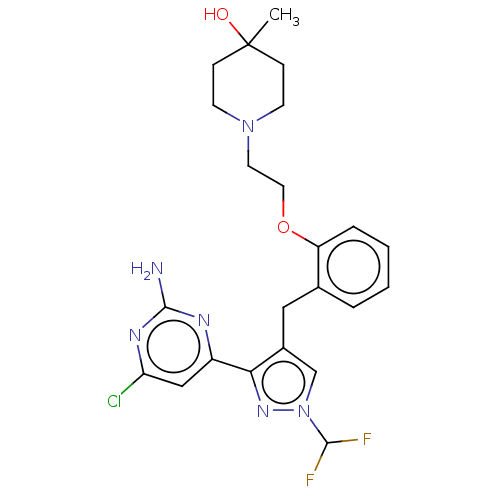
(CHEMBL5218702)Show SMILES CC1(O)CCN(CCOc2ccccc2Cc2cn(nc2-c2cc(Cl)nc(N)n2)C(F)F)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50180269
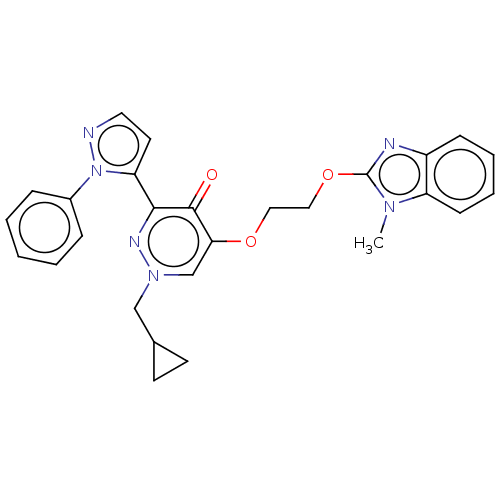
(CHEMBL3814367)Show SMILES Cn1c(OCCOc2cn(CC3CC3)nc(-c3ccnn3-c3ccccc3)c2=O)nc2ccccc12 Show InChI InChI=1S/C27H26N6O3/c1-31-22-10-6-5-9-21(22)29-27(31)36-16-15-35-24-18-32(17-19-11-12-19)30-25(26(24)34)23-13-14-28-33(23)20-7-3-2-4-8-20/h2-10,13-14,18-19H,11-12,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human full length PDE10A2 expressed in African green monkey COS7 cells using [3H]cGMP as substrate preincubated for 30 mins followed by... |
Bioorg Med Chem 24: 3447-55 (2016)
Article DOI: 10.1016/j.bmc.2016.05.049
BindingDB Entry DOI: 10.7270/Q2H9974G |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607685
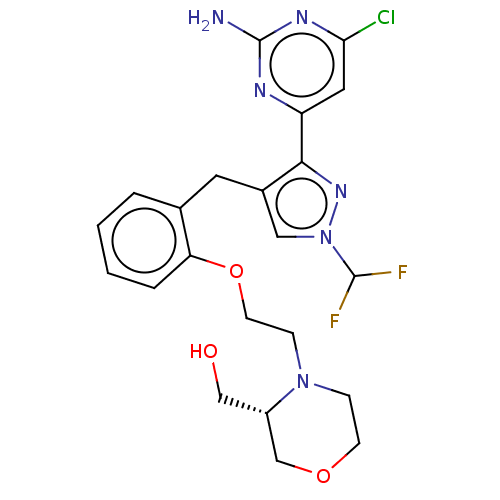
(CHEMBL5220647)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524978
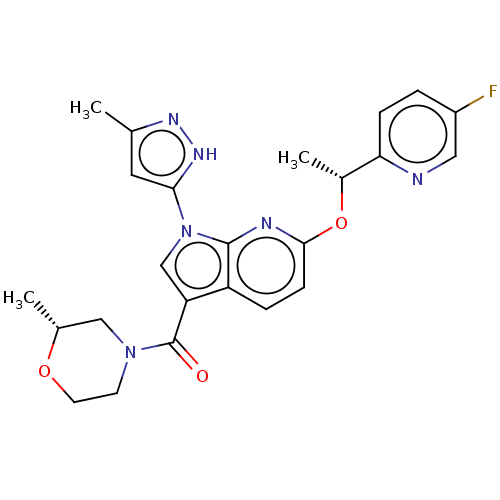
(CHEMBL4573505)Show SMILES C[C@@H](Oc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N1CCO[C@H](C)C1)c1ccc(F)cn1 |r| Show InChI InChI=1S/C24H25FN6O3/c1-14-10-21(29-28-14)31-13-19(24(32)30-8-9-33-15(2)12-30)18-5-7-22(27-23(18)31)34-16(3)20-6-4-17(25)11-26-20/h4-7,10-11,13,15-16H,8-9,12H2,1-3H3,(H,28,29)/t15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459648
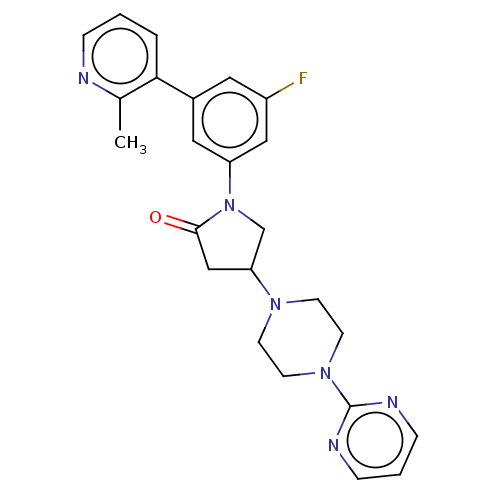
(CHEMBL4210423)Show SMILES Cc1ncccc1-c1cc(F)cc(c1)N1CC(CC1=O)N1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C24H25FN6O/c1-17-22(4-2-5-26-17)18-12-19(25)14-20(13-18)31-16-21(15-23(31)32)29-8-10-30(11-9-29)24-27-6-3-7-28-24/h2-7,12-14,21H,8-11,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607684
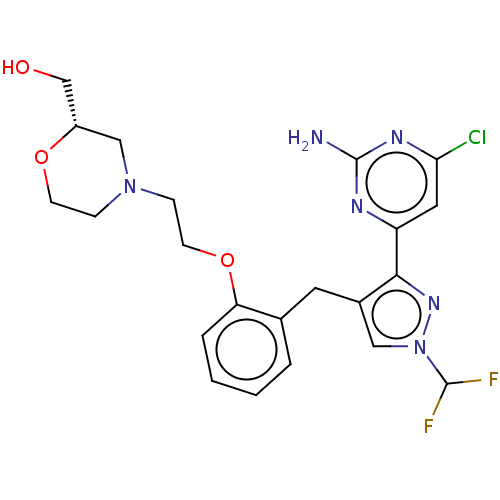
(CHEMBL5219443)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607686

(CHEMBL5219830)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607678

(CHEMBL5218878)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCNC(=O)C1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524981

(CHEMBL4562879)Show SMILES C[C@H](Nc1ccc2ncc(-c3cc(C)n[nH]3)n2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C18H16F2N6/c1-10-7-15(24-23-10)16-9-21-18-6-5-17(25-26(16)18)22-11(2)13-4-3-12(19)8-14(13)20/h3-9,11H,1-2H3,(H,22,25)(H,23,24)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524988
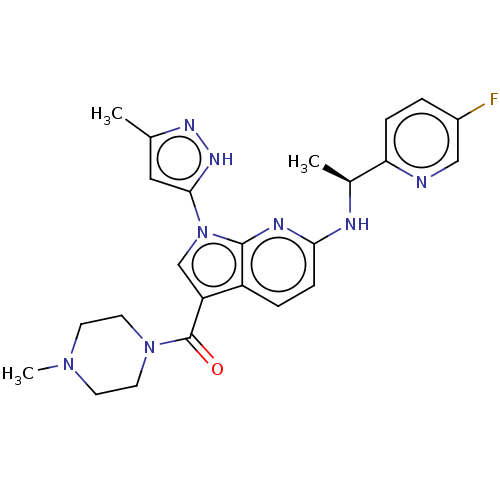
(CHEMBL4586773)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N1CCN(C)CC1)c1ccc(F)cn1 |r| Show InChI InChI=1S/C24H27FN8O/c1-15-12-22(30-29-15)33-14-19(24(34)32-10-8-31(3)9-11-32)18-5-7-21(28-23(18)33)27-16(2)20-6-4-17(25)13-26-20/h4-7,12-14,16H,8-11H2,1-3H3,(H,27,28)(H,29,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524975

(CHEMBL4476859)Show SMILES CC(Oc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N1CCOCC1)c1ccc(F)cn1 Show InChI InChI=1S/C23H23FN6O3/c1-14-11-20(28-27-14)30-13-18(23(31)29-7-9-32-10-8-29)17-4-6-21(26-22(17)30)33-15(2)19-5-3-16(24)12-25-19/h3-6,11-13,15H,7-10H2,1-2H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459636
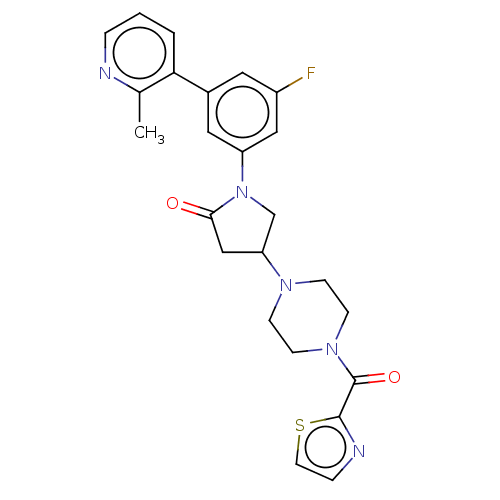
(CHEMBL4205550)Show SMILES Cc1ncccc1-c1cc(F)cc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C24H24FN5O2S/c1-16-21(3-2-4-26-16)17-11-18(25)13-19(12-17)30-15-20(14-22(30)31)28-6-8-29(9-7-28)24(32)23-27-5-10-33-23/h2-5,10-13,20H,6-9,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607673
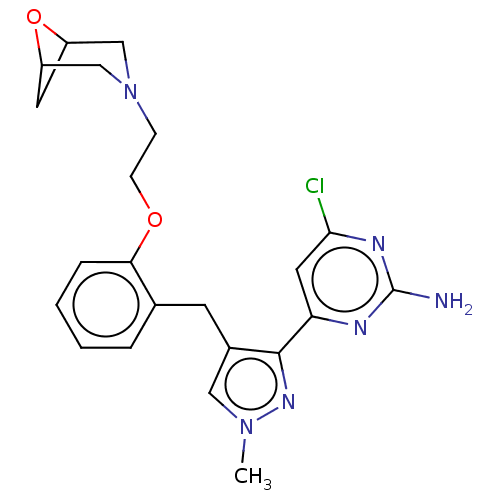
(CHEMBL5220895)Show SMILES Cn1cc(Cc2ccccc2OCCN2CC3CC(C2)O3)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607674
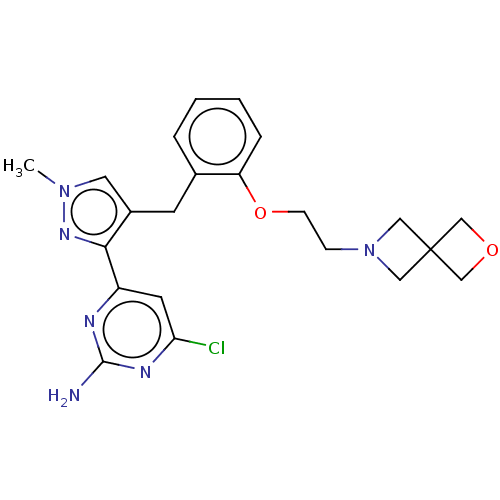
(CHEMBL5220347)Show SMILES Cn1cc(Cc2ccccc2OCCN2CC3(COC3)C2)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459646
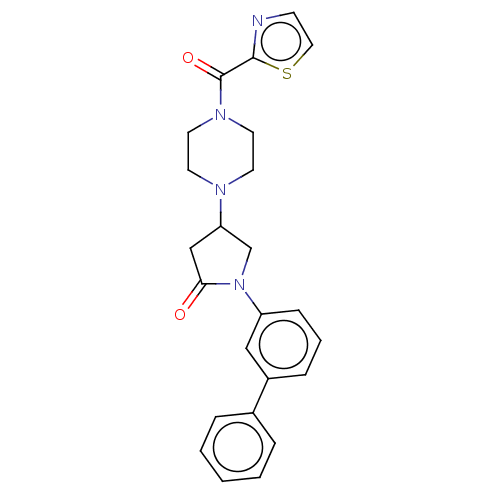
(CHEMBL4211832)Show SMILES O=C(N1CCN(CC1)C1CN(C(=O)C1)c1cccc(c1)-c1ccccc1)c1nccs1 Show InChI InChI=1S/C24H24N4O2S/c29-22-16-21(26-10-12-27(13-11-26)24(30)23-25-9-14-31-23)17-28(22)20-8-4-7-19(15-20)18-5-2-1-3-6-18/h1-9,14-15,21H,10-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459650
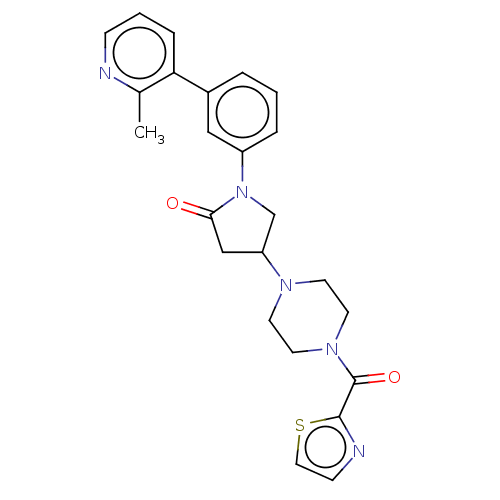
(CHEMBL4217781)Show SMILES Cc1ncccc1-c1cccc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C24H25N5O2S/c1-17-21(6-3-7-25-17)18-4-2-5-19(14-18)29-16-20(15-22(29)30)27-9-11-28(12-10-27)24(31)23-26-8-13-32-23/h2-8,13-14,20H,9-12,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524983

(CHEMBL4458269)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C1=CCOCC1)c1ccc(F)cn1 |r,t:21| Show InChI InChI=1S/C23H23FN6O/c1-14-11-22(29-28-14)30-13-19(16-7-9-31-10-8-16)18-4-6-21(27-23(18)30)26-15(2)20-5-3-17(24)12-25-20/h3-7,11-13,15H,8-10H2,1-2H3,(H,26,27)(H,28,29)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524977
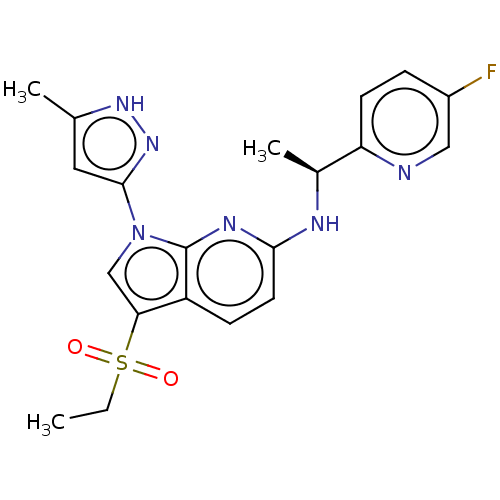
(CHEMBL4443254)Show SMILES CCS(=O)(=O)c1cn(-c2cc(C)[nH]n2)c2nc(N[C@@H](C)c3ccc(F)cn3)ccc12 |r| Show InChI InChI=1S/C20H21FN6O2S/c1-4-30(28,29)17-11-27(19-9-12(2)25-26-19)20-15(17)6-8-18(24-20)23-13(3)16-7-5-14(21)10-22-16/h5-11,13H,4H2,1-3H3,(H,23,24)(H,25,26)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459634
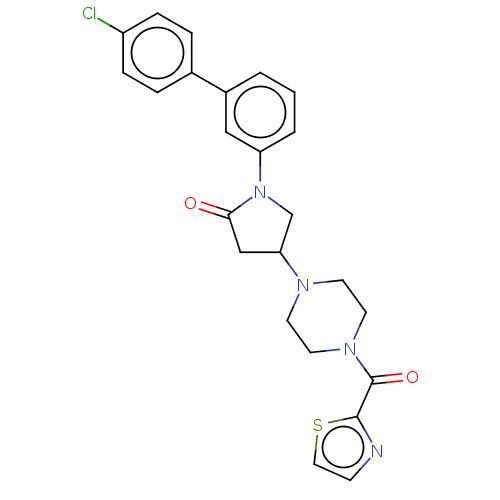
(CHEMBL4216838)Show SMILES Clc1ccc(cc1)-c1cccc(c1)N1CC(CC1=O)N1CCN(CC1)C(=O)c1nccs1 Show InChI InChI=1S/C24H23ClN4O2S/c25-19-6-4-17(5-7-19)18-2-1-3-20(14-18)29-16-21(15-22(29)30)27-9-11-28(12-10-27)24(31)23-26-8-13-32-23/h1-8,13-14,21H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524982
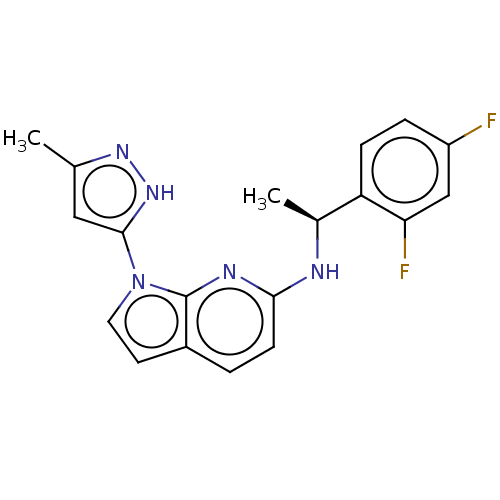
(CHEMBL4448434)Show SMILES C[C@H](Nc1ccc2ccn(-c3cc(C)n[nH]3)c2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C19H17F2N5/c1-11-9-18(25-24-11)26-8-7-13-3-6-17(23-19(13)26)22-12(2)15-5-4-14(20)10-16(15)21/h3-10,12H,1-2H3,(H,22,23)(H,24,25)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524984

(CHEMBL4434659)Show SMILES COCc1cn(-c2cc(C)n[nH]2)c2nc(OC(C)c3ccc(F)cn3)ccc12 Show InChI InChI=1S/C20H20FN5O2/c1-12-8-18(25-24-12)26-10-14(11-27-3)16-5-7-19(23-20(16)26)28-13(2)17-6-4-15(21)9-22-17/h4-10,13H,11H2,1-3H3,(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50459631
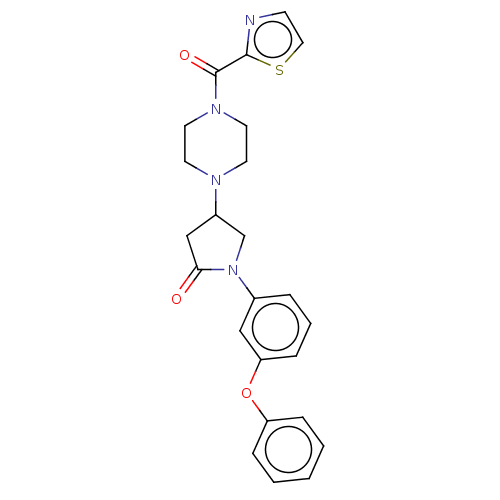
(CHEMBL4212945)Show SMILES O=C(N1CCN(CC1)C1CN(C(=O)C1)c1cccc(Oc2ccccc2)c1)c1nccs1 Show InChI InChI=1S/C24H24N4O3S/c29-22-16-19(26-10-12-27(13-11-26)24(30)23-25-9-14-32-23)17-28(22)18-5-4-8-21(15-18)31-20-6-2-1-3-7-20/h1-9,14-15,19H,10-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MAGL expressed in Escherichia coli BL21(DE3) assessed as reduction in arachidonic acid production from 2-arachidonoylg... |
J Med Chem 61: 9205-9217 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00824
BindingDB Entry DOI: 10.7270/Q2FR008T |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607685

(CHEMBL5220647)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data