

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human N-terminal GST-fusion tagged TRKB kinase domain (456 to 822 residues) expressed in baculovirus expression system by HTR... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160321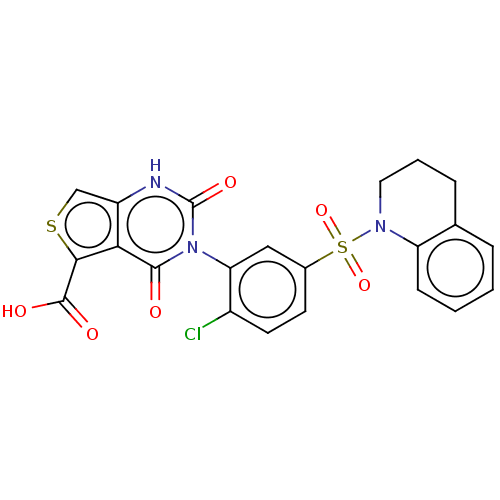 (US9040693, 22) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human His-tagged TRKA kinase domain (441 to 796 residues) expressed in baculovirus expression system by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of TRKA E735A mutant (unknown origin) expressed in baculovirus infected sf9 cells assessed as kinase domain dimerization by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559528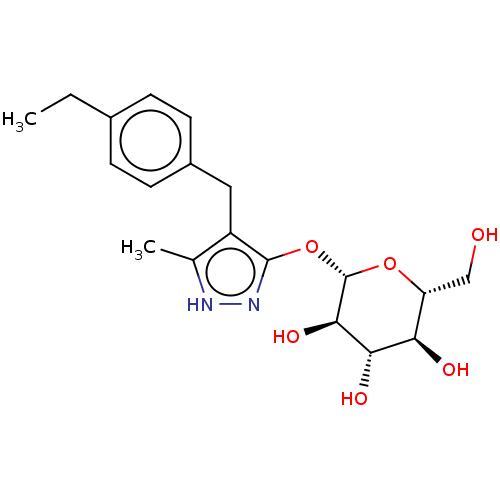 (CHEMBL3950588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559538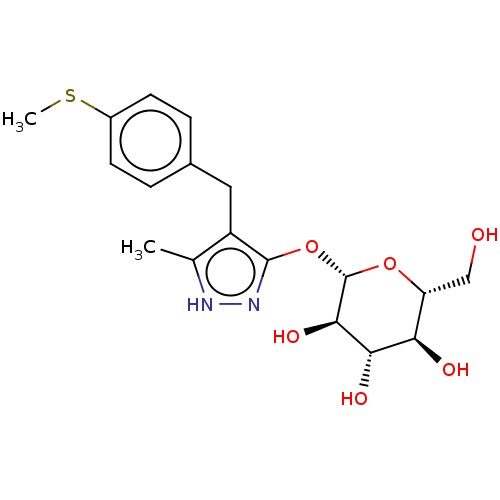 (CHEMBL3908950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50394556 (CHEMBL2159100) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction of [14C]-AMG uptake | Bioorg Med Chem 20: 6598-612 (2012) Article DOI: 10.1016/j.bmc.2012.09.037 BindingDB Entry DOI: 10.7270/Q26D5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50426906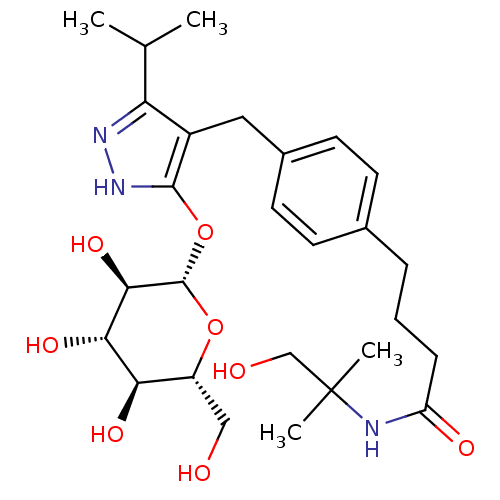 (CHEMBL2323692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559527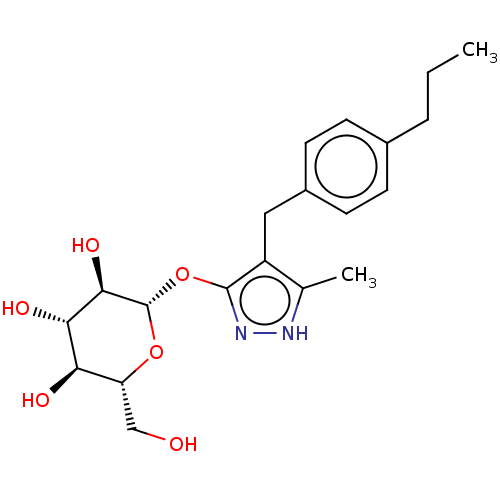 (CHEMBL3907970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559522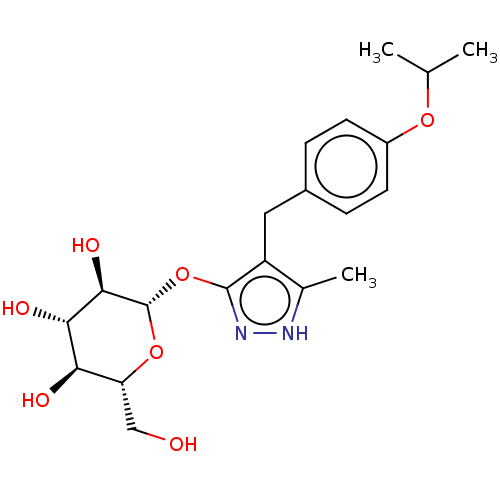 (CHEMBL3960449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426894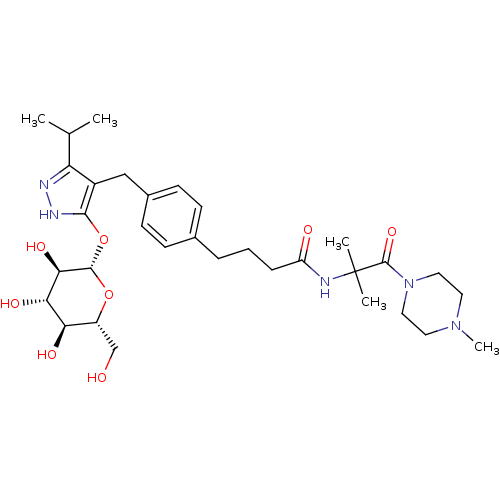 (CHEMBL2323684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160326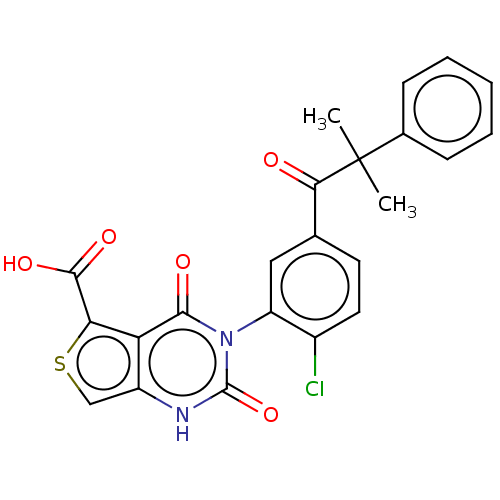 (US9040693, 146) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426887 (CHEMBL2323695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559524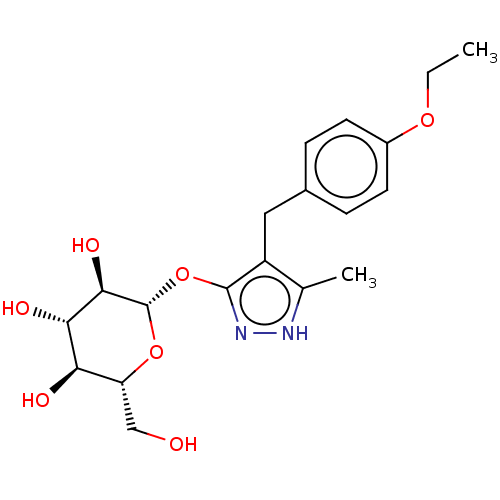 (CHEMBL3923809) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559525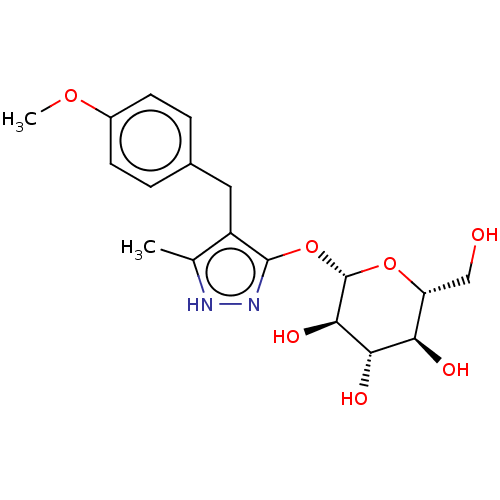 (CHEMBL3934976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559529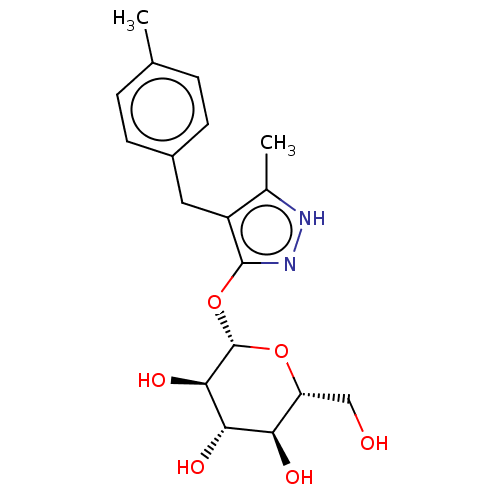 (CHEMBL3896936) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426906 (CHEMBL2323692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160329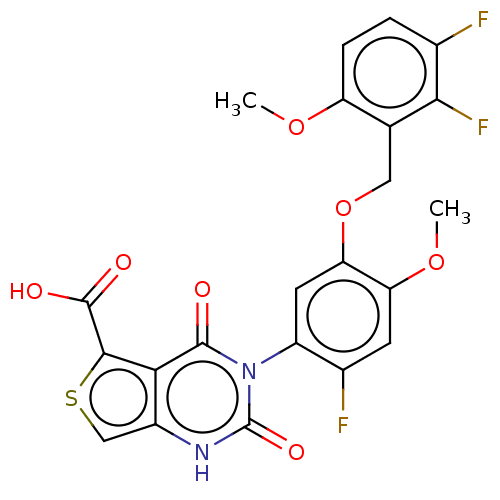 (US9040693, 233) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426895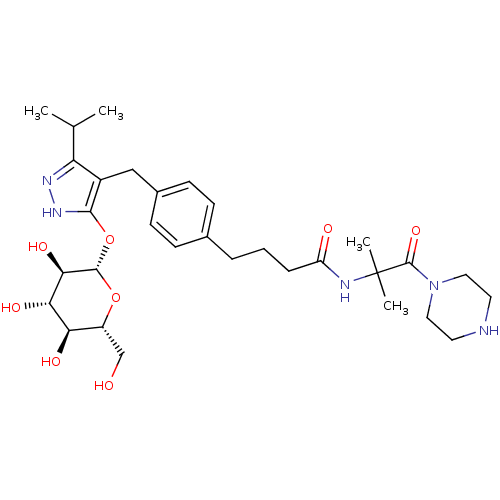 (CHEMBL2323683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160330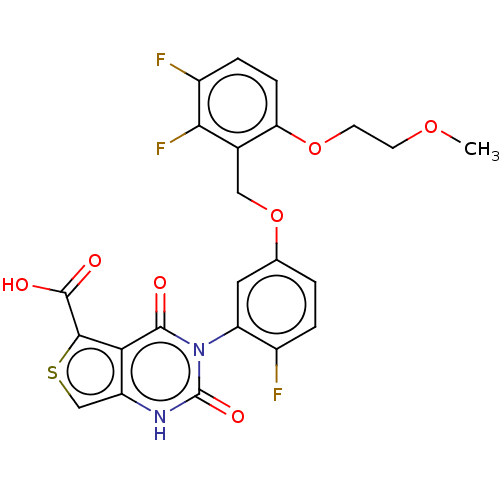 (US9040693, 367) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559523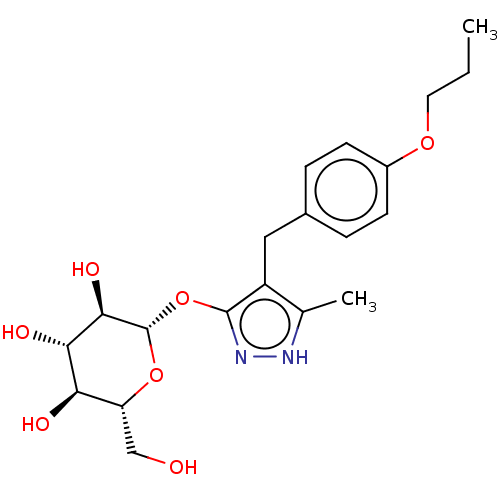 (CHEMBL3915296) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559539 (CHEMBL3980689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559526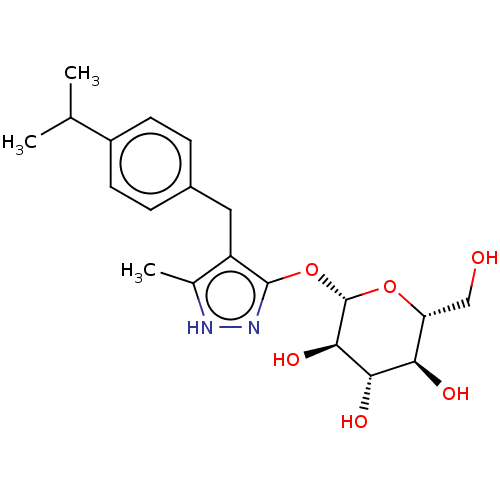 (CHEMBL3969998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160327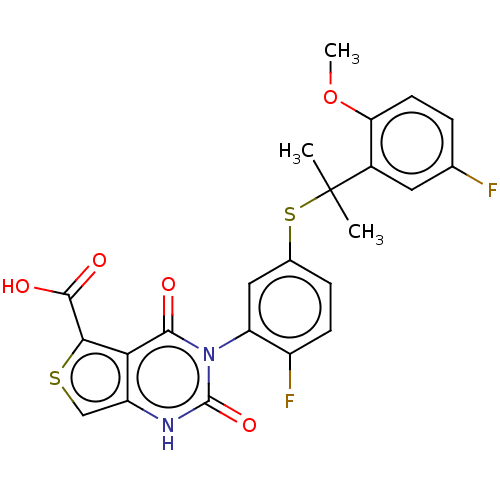 (US9040693, 191) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50426894 (CHEMBL2323684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559517 (Remogliflozin | Remogliflozin a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160325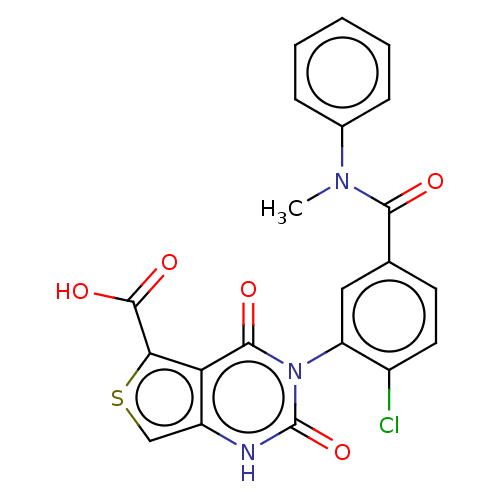 (US9040693, 95) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160328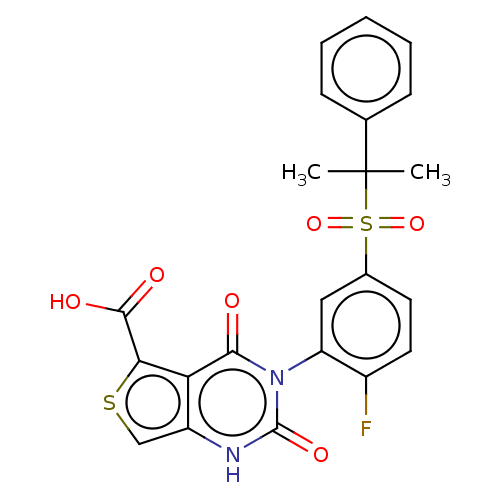 (US9040693, 202) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50426895 (CHEMBL2323683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Rattus norvegicus) | BDBM50426887 (CHEMBL2323695) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of rat SGLT1 | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426889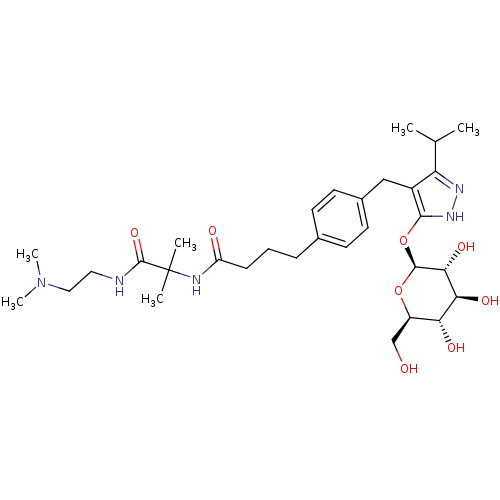 (CHEMBL2323409) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426893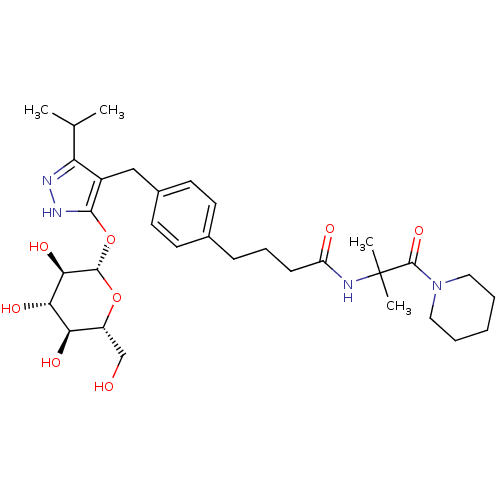 (CHEMBL2323405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160332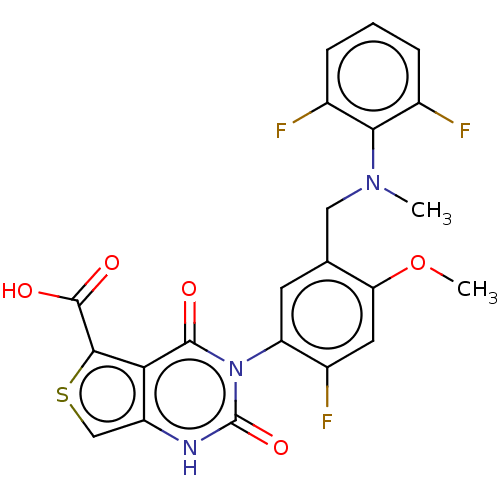 (US9040693, 420) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160324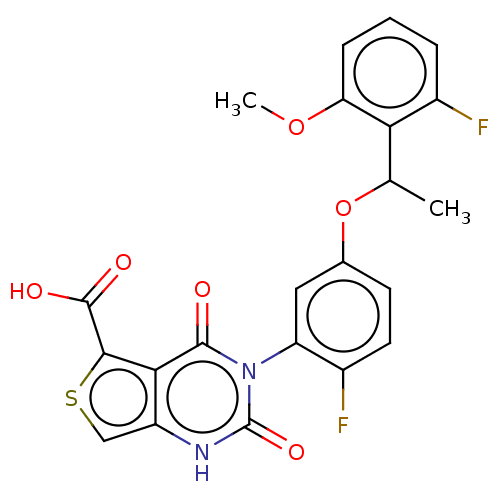 (US9040693, 48) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50394532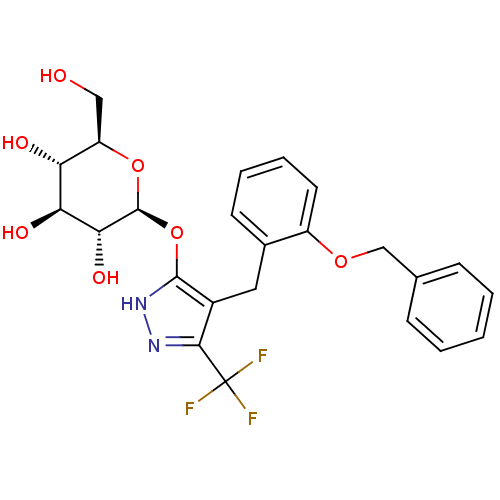 (CHEMBL2159094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as reduction of [14C]-AMG uptake | Bioorg Med Chem 20: 6598-612 (2012) Article DOI: 10.1016/j.bmc.2012.09.037 BindingDB Entry DOI: 10.7270/Q26D5V36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20875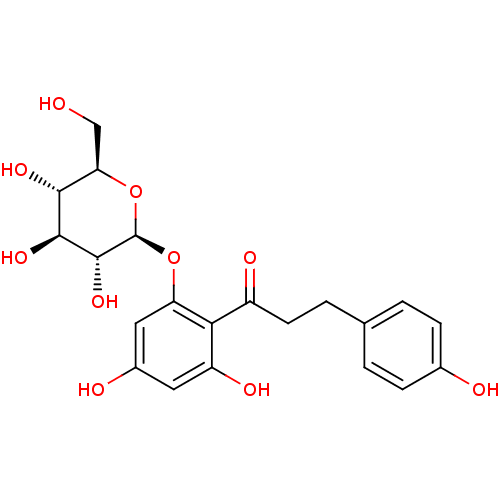 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction of [14C]-AMG uptake | Bioorg Med Chem 20: 6598-612 (2012) Article DOI: 10.1016/j.bmc.2012.09.037 BindingDB Entry DOI: 10.7270/Q26D5V36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160331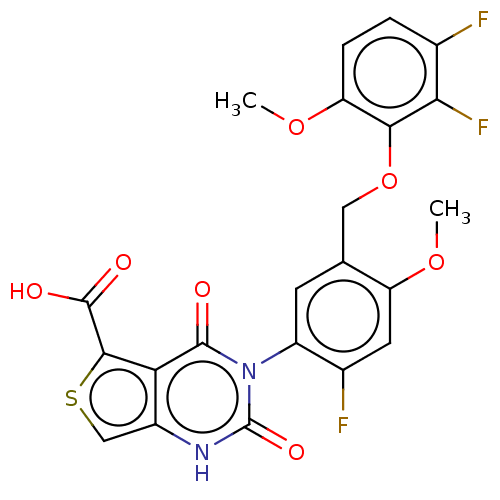 (US9040693, 414) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50426893 (CHEMBL2323405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559519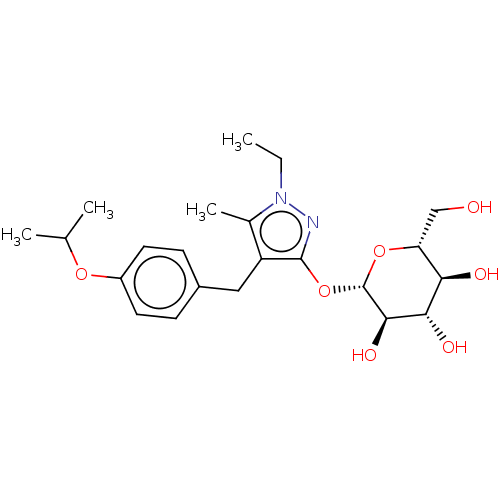 (CHEMBL4786882) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426905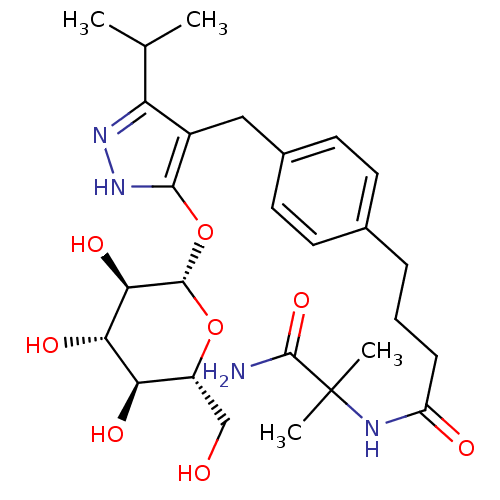 (CHEMBL2323693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50426889 (CHEMBL2323409) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50426888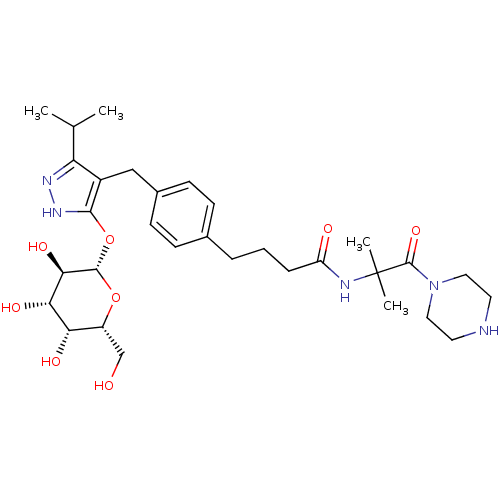 (CHEMBL2323694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS7 cells assessed as inhibition [14C]-AMG cellular uptake | Bioorg Med Chem 21: 748-65 (2013) Article DOI: 10.1016/j.bmc.2012.11.041 BindingDB Entry DOI: 10.7270/Q2445NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559537 (CHEMBL3972664) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SGLT2 expressed in COS7 cells assessed as reduction in [14C]-AMG uptake | Citation and Details Article DOI: 10.1016/j.bmc.2021.116033 BindingDB Entry DOI: 10.7270/Q29G5RHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 270 total ) | Next | Last >> |