

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163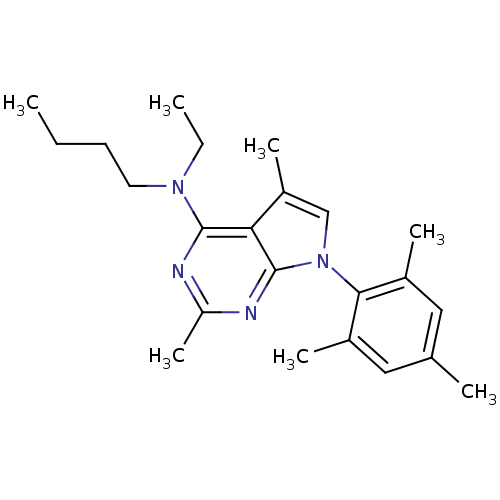 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against [125I]-Try0-o-Corticotropin-releasing Factor to Corticotropin releasing hormone receptor 1 from ovine | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 2 (RAT) | BDBM50058163 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-Tyr0-sauvagine to Corticotropin releasing hormone receptor 2 (CRF2) | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246465 (US9447114, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246466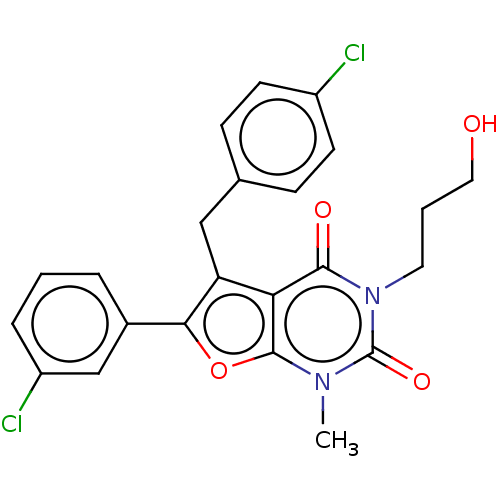 (US9447114, 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246446 (US9447114, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246445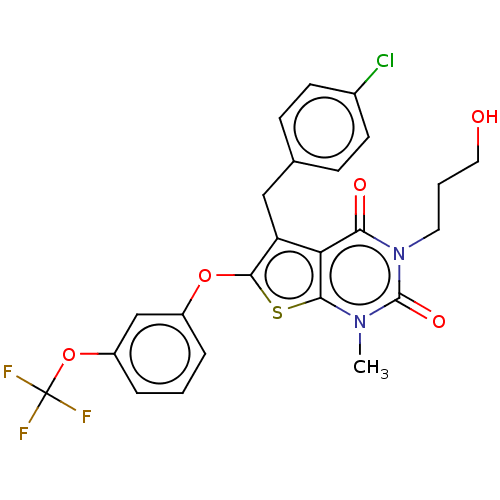 (US9447114, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246463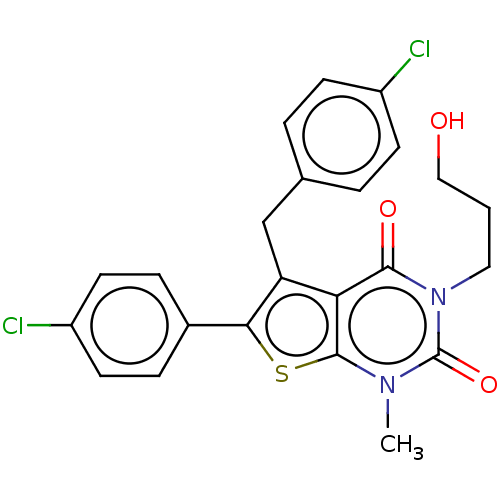 (US9447114, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246465 (US9447114, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20954 (2-(4-methoxy-2,6-dimethylphenoxy)-3,6-dimethyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20950 (2-(4-bromo-2,6-dimethylphenoxy)-3,6-dimethyl-4-(pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20959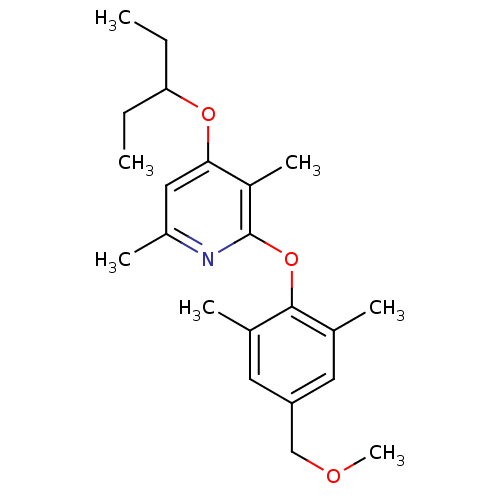 (2-Aryloxy-4-alkoxy-pyridine, 40 | 2-[4-(methoxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20971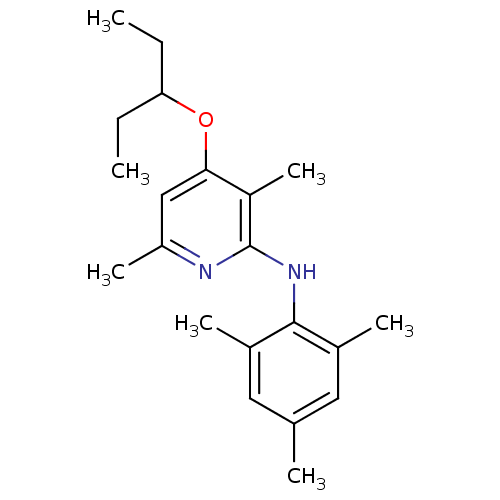 (2-Arylamino-4-alkyloxypyridine, 13 | 3,6-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20966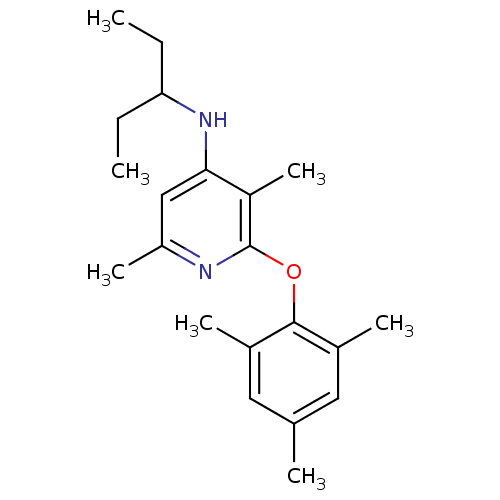 (2-Aryloxy-4-alkylaminopyridine, 3a | 3,6-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50058163 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of binding of 70 pM [125I]-Try0-o-Corticotropin-releasing Factor (CFR) to Corticotropin releasing hormone receptor 1 in Rat brain membrane | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20949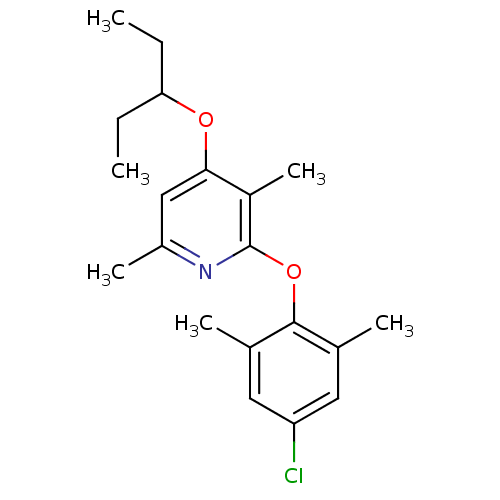 (2-(4-chloro-2,6-dimethylphenoxy)-3,6-dimethyl-4-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20968 (2-(4-bromo-2,6-dimethylphenoxy)-3,6-dimethyl-N-(pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20969 (2-Aryloxy-4-alkylaminopyridine, 5a | methyl 2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20933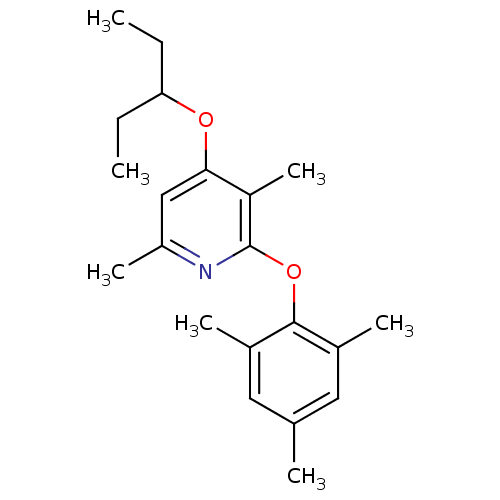 (3,6-dimethyl-4-(pentan-3-yloxy)-2-(2,4,6-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20933 (3,6-dimethyl-4-(pentan-3-yloxy)-2-(2,4,6-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20967 (2-(4-chloro-2,6-dimethylphenoxy)-3,6-dimethyl-N-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50058164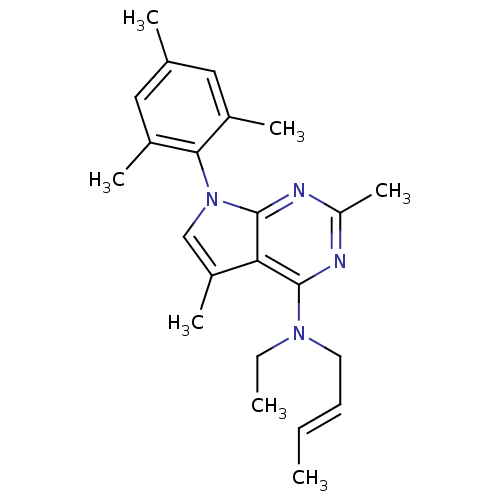 (But-2-enyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of binding of 70 pM [125I]-Try0-o-Corticotropin-releasing Factor (CFR) to Corticotropin releasing hormone receptor 1 in Rat brain membrane | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20935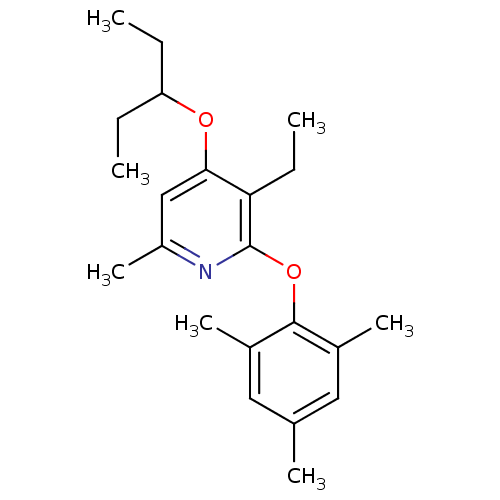 (2-Aryloxy-4-alkoxy-pyridine, 16 | 3-ethyl-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20947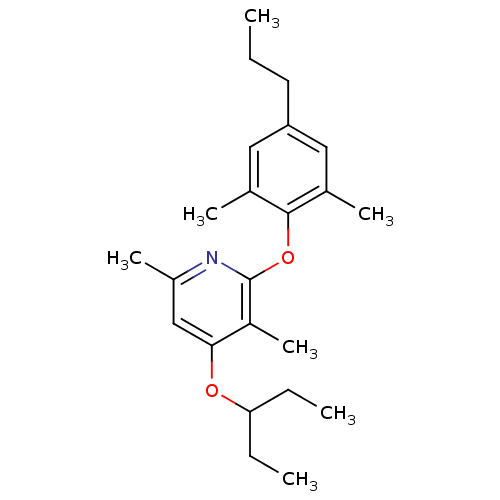 (2-(2,6-dimethyl-4-propylphenoxy)-3,6-dimethyl-4-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20941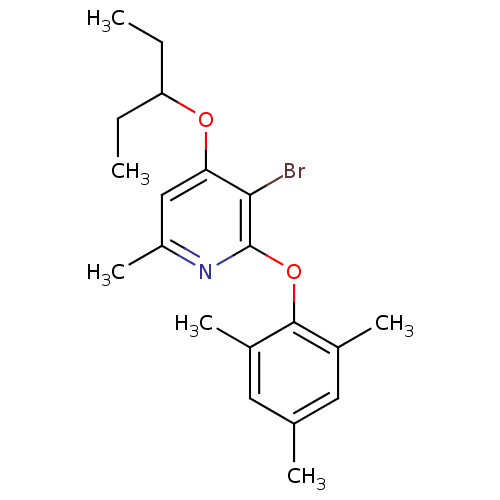 (2-Aryloxy-4-alkoxy-pyridine, 22 | 3-bromo-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20965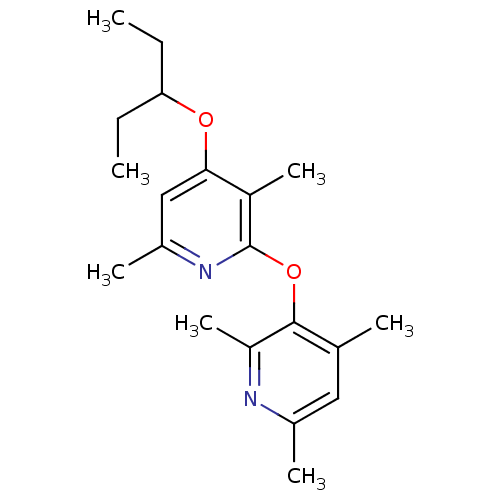 (2-pyridinoxy-4-alkoxy-pyridine, 14 | 3,6-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20962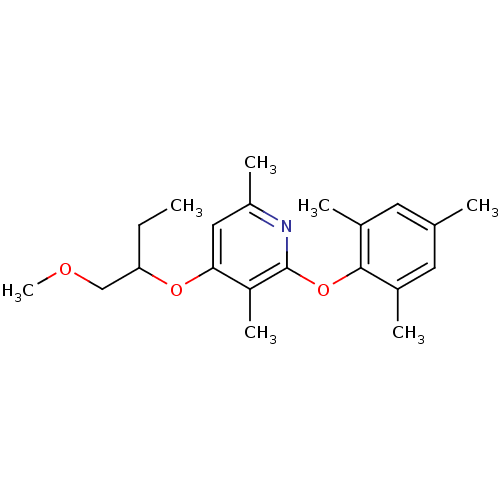 (2-Aryloxy-4-alkoxy-pyridine, 43 | 4-[(1-methoxybut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20946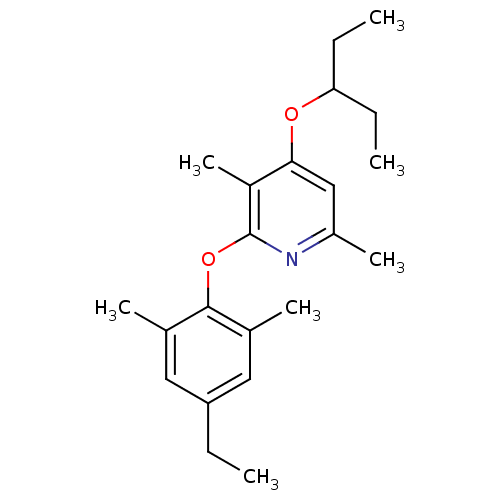 (2-(4-ethyl-2,6-dimethylphenoxy)-3,6-dimethyl-4-(pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20951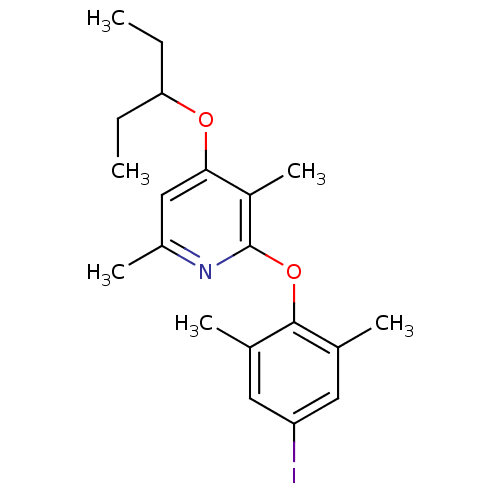 (2-(4-iodo-2,6-dimethylphenoxy)-3,6-dimethyl-4-(pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20939 (2-Aryloxy-4-alkoxy-pyridine, 20 | 6-methyl-4-(pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20938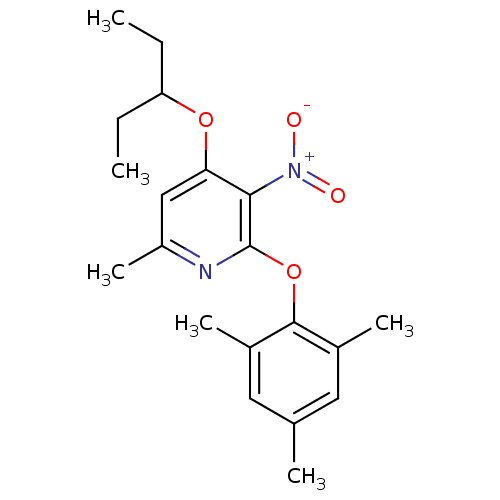 (2-Aryloxy-4-alkoxy-pyridine, 19 | 6-methyl-3-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246464 (US9447114, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hydra Biosciences, Inc. US Patent | Assay Description TRPC5 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separation) on gla... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20964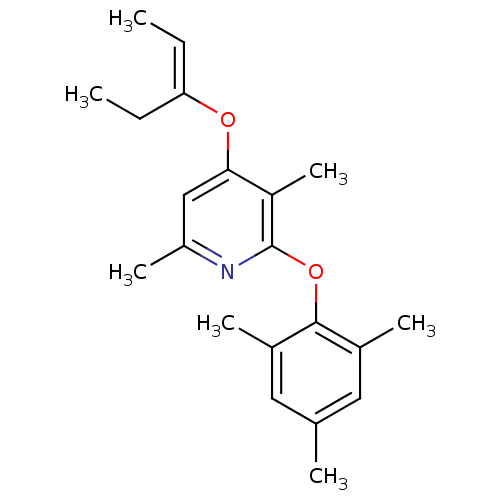 (2-Aryloxy-4-alkoxy-pyridine, 13 | 3,6-dimethyl-4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20952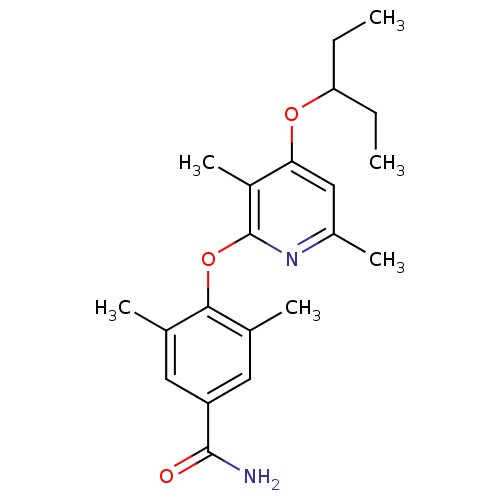 (2-Aryloxy-4-alkoxy-pyridine, 33 | 4-{[3,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20955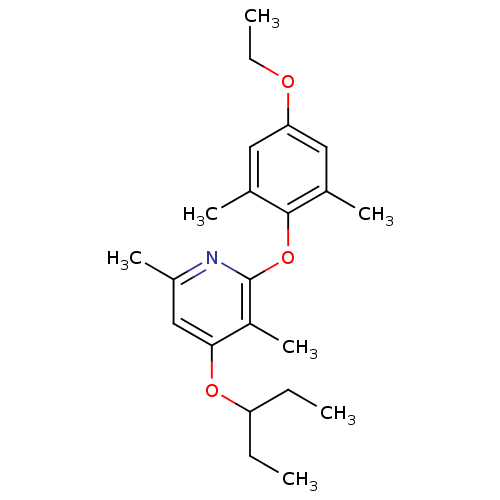 (2-(4-ethoxy-2,6-dimethylphenoxy)-3,6-dimethyl-4-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246453 (US9447114, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <21 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20940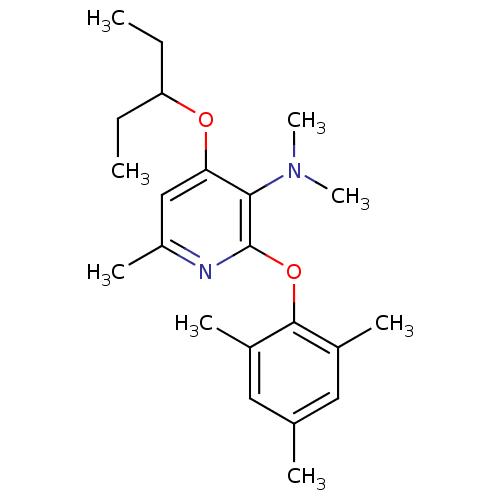 (2-Aryloxy-4-alkoxy-pyridine, 21 | N,N,6-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246462 (US9447114, 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246458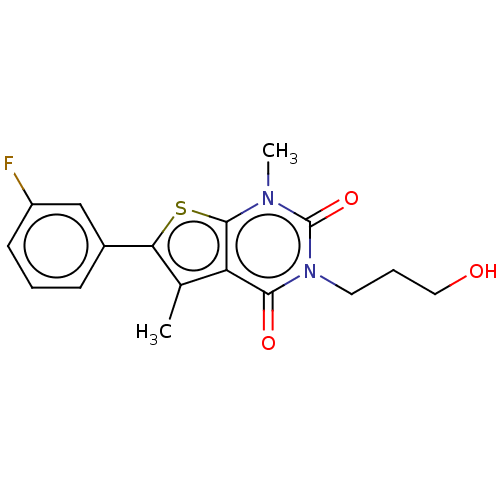 (US9447114, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246450 (US9447114, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246456 (US9447114, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246449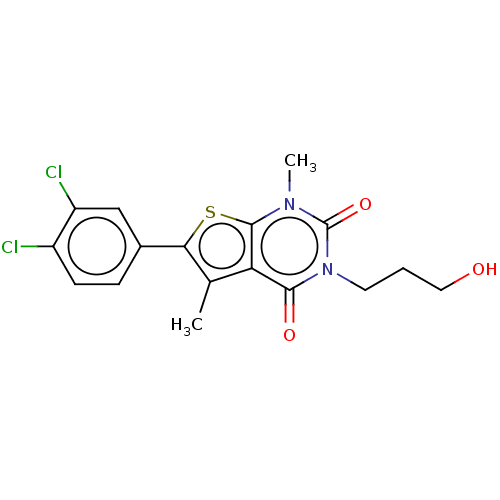 (US9447114, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <27 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20961 (2-Aryloxy-4-alkoxy-pyridine, 42 | 4-(butan-2-yloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20970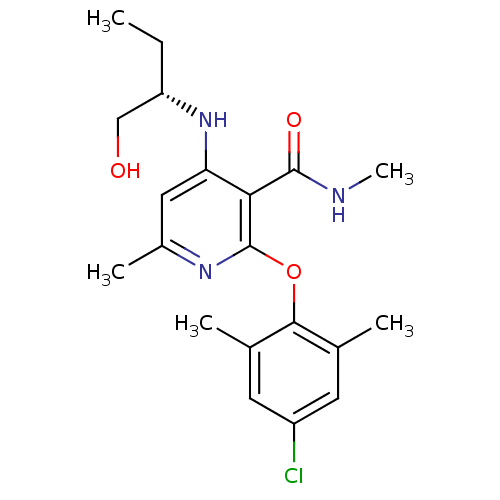 (2-(4-chloro-2,6-dimethylphenoxy)-4-{[(2S)-1-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1385-92 (2008) Article DOI: 10.1021/jm070579c BindingDB Entry DOI: 10.7270/Q29C6VQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246457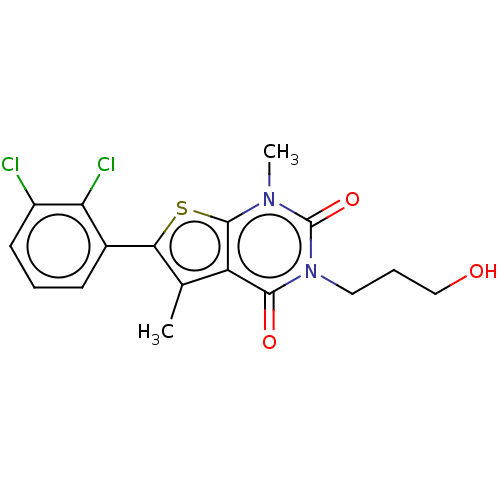 (US9447114, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20948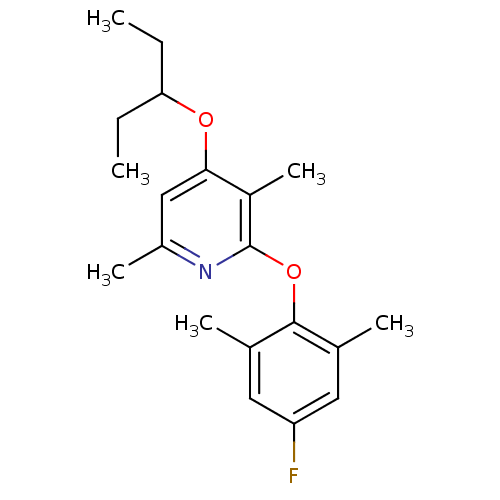 (2-(4-fluoro-2,6-dimethylphenoxy)-3,6-dimethyl-4-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246451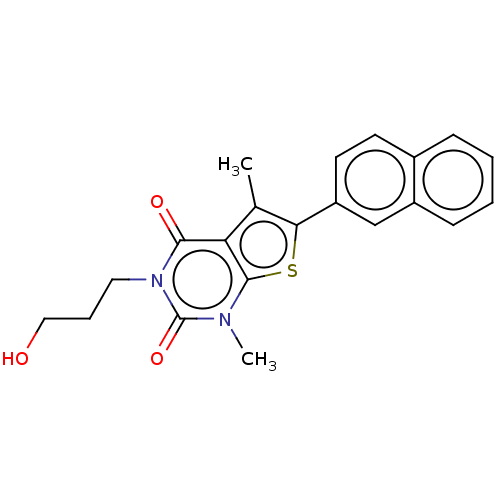 (US9447114, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34.9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20957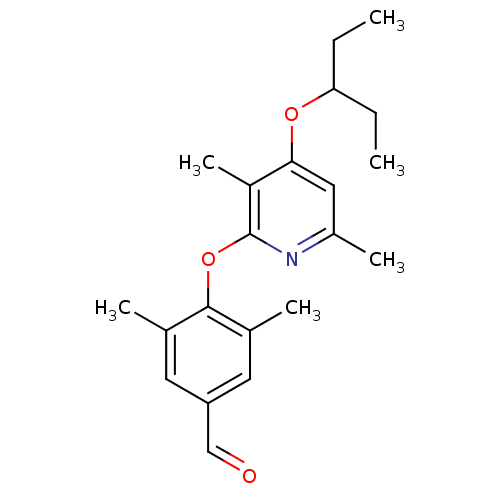 (2-Aryloxy-4-alkoxy-pyridine, 38 | 4-{[3,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20960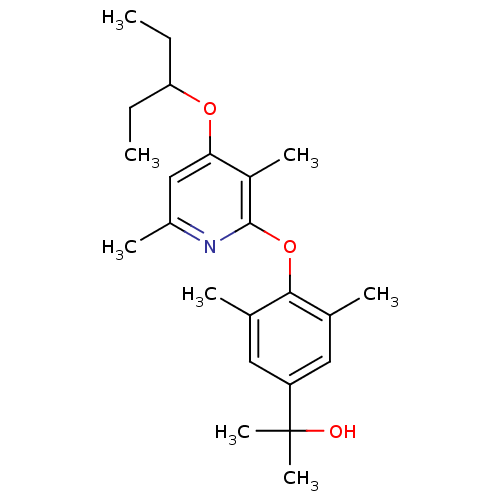 (2-(4-{[3,6-dimethyl-4-(pentan-3-yloxy)pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 5 (Homo sapiens (Human)) | BDBM246448 (US9447114, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Hydra Biosciences, Inc. US Patent | Assay Description The commercially available HEK293/TREx line (Invitrogen) was stably transfected with a TRPC5 construct and screened by conventional calcium imaging t... | US Patent US9447114 (2016) BindingDB Entry DOI: 10.7270/Q2ST7NRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20958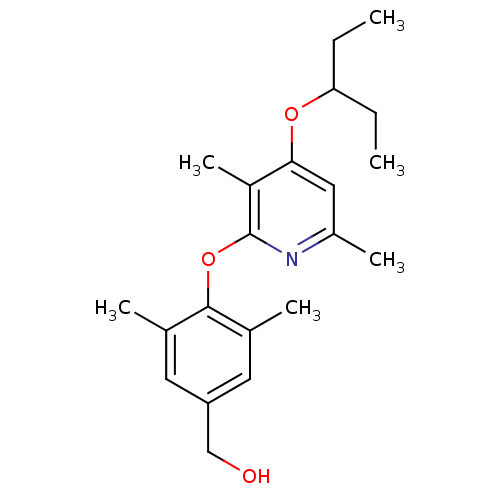 ((4-{[3,6-dimethyl-4-(pentan-3-yloxy)pyridin-2-yl]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |