 Found 620 hits with Last Name = 'ghirmai' and Initial = 's'
Found 620 hits with Last Name = 'ghirmai' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304170
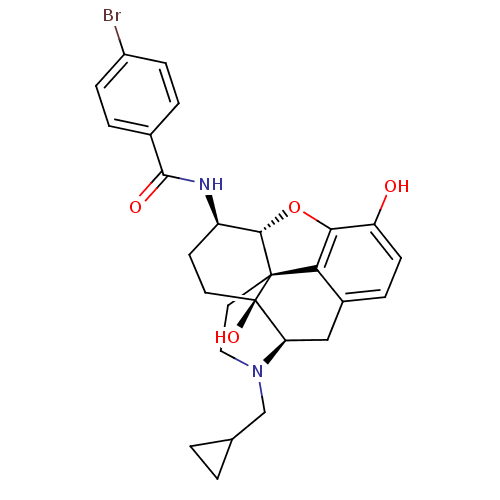
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C27H29BrN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50378578

(CHEMBL1627119)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3S/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(26-9)1-25-29(19,20)27-30(21,22)28-31(23,24)32/h2-3,5-6,9,16-17H,1H2,(H,19,20)(H,21,22)(H2,23,24,32)(H3,11,13,14,18)/p-4/t3-,5-,6-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304175
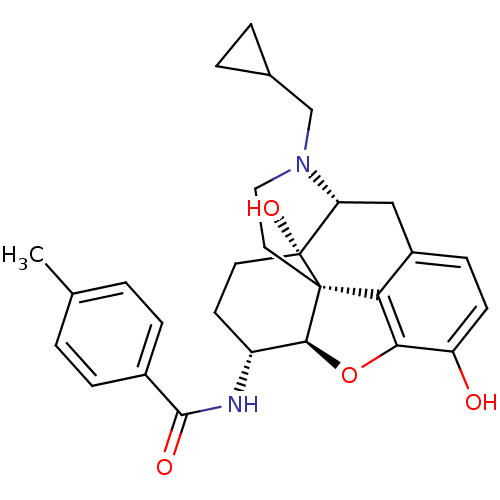
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Cc1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C28H32N2O4/c1-16-2-6-18(7-3-16)26(32)29-20-10-11-28(33)22-14-19-8-9-21(31)24-23(19)27(28,25(20)34-24)12-13-30(22)15-17-4-5-17/h2-3,6-9,17,20,22,25,31,33H,4-5,10-15H2,1H3,(H,29,32)/t20-,22-,25+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86936
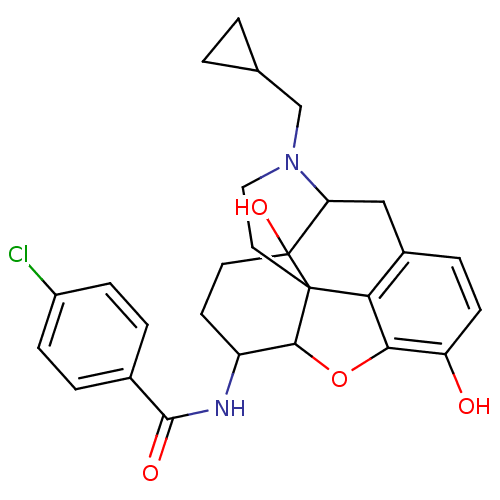
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccc(Cl)cc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H29ClN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86932
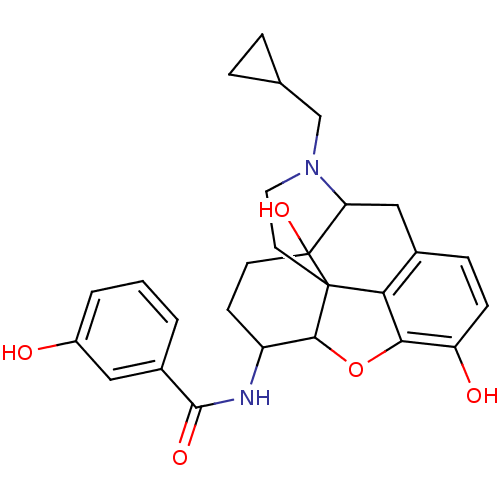
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1cccc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:12:13:28.27.26:16.17.33,THB:14:13:28.27.26:16.17.33| Show InChI InChI=1S/C27H30N2O5/c30-18-3-1-2-17(12-18)25(32)28-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-29(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,30-31,33H,4-5,8-11,13-14H2,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86937
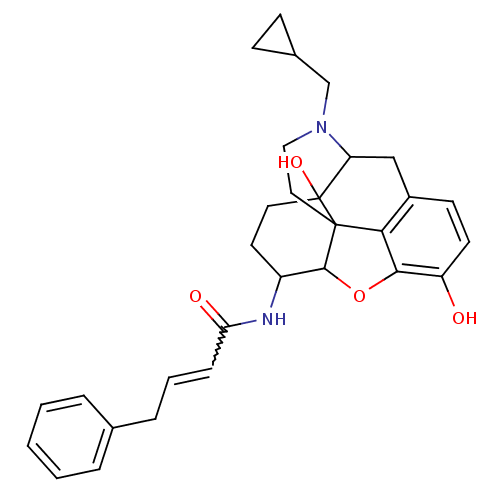
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)C=CCc1ccccc1 |w:27.32,TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C30H34N2O4/c33-23-12-11-21-17-24-30(35)14-13-22(31-25(34)8-4-7-19-5-2-1-3-6-19)28-29(30,26(21)27(23)36-28)15-16-32(24)18-20-9-10-20/h1-6,8,11-12,20,22,24,28,33,35H,7,9-10,13-18H2,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86928

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)CCc1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C29H34N2O4/c32-22-10-9-20-16-23-29(34)13-12-21(30-24(33)11-8-18-4-2-1-3-5-18)27-28(29,25(20)26(22)35-27)14-15-31(23)17-19-6-7-19/h1-5,9-10,19,21,23,27,32,34H,6-8,11-17H2,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86944
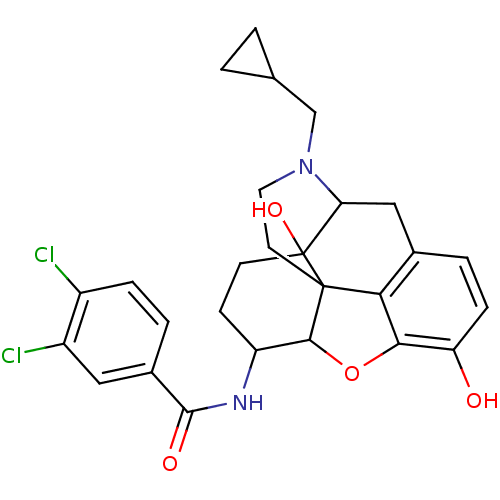
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccc(Cl)c(Cl)c1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H28Cl2N2O4/c28-17-5-3-16(11-18(17)29)25(33)30-19-7-8-27(34)21-12-15-4-6-20(32)23-22(15)26(27,24(19)35-23)9-10-31(21)13-14-1-2-14/h3-6,11,14,19,21,24,32,34H,1-2,7-10,12-13H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86944

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccc(Cl)c(Cl)c1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H28Cl2N2O4/c28-17-5-3-16(11-18(17)29)25(33)30-19-7-8-27(34)21-12-15-4-6-20(32)23-22(15)26(27,24(19)35-23)9-10-31(21)13-14-1-2-14/h3-6,11,14,19,21,24,32,34H,1-2,7-10,12-13H2,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50304175

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Cc1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C28H32N2O4/c1-16-2-6-18(7-3-16)26(32)29-20-10-11-28(33)22-14-19-8-9-21(31)24-23(19)27(28,25(20)34-24)12-13-30(22)15-17-4-5-17/h2-3,6-9,17,20,22,25,31,33H,4-5,10-15H2,1H3,(H,29,32)/t20-,22-,25+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304175

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Cc1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C28H32N2O4/c1-16-2-6-18(7-3-16)26(32)29-20-10-11-28(33)22-14-19-8-9-21(31)24-23(19)27(28,25(20)34-24)12-13-30(22)15-17-4-5-17/h2-3,6-9,17,20,22,25,31,33H,4-5,10-15H2,1H3,(H,29,32)/t20-,22-,25+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86929
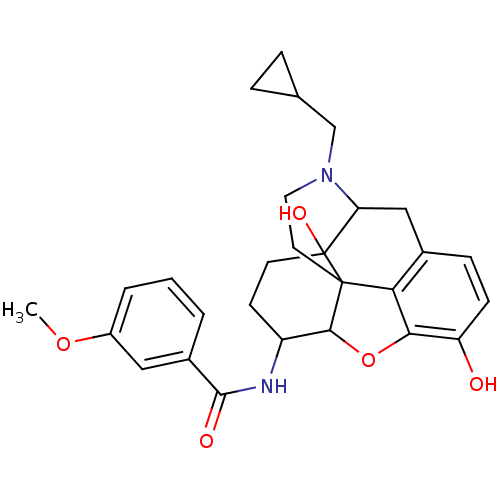
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cccc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:15:14:17.18.34:27.28.29,13:14:17.18.34:27.28.29| Show InChI InChI=1S/C28H32N2O5/c1-34-19-4-2-3-18(13-19)26(32)29-20-9-10-28(33)22-14-17-7-8-21(31)24-23(17)27(28,25(20)35-24)11-12-30(22)15-16-5-6-16/h2-4,7-8,13,16,20,22,25,31,33H,5-6,9-12,14-15H2,1H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86928

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)CCc1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C29H34N2O4/c32-22-10-9-20-16-23-29(34)13-12-21(30-24(33)11-8-18-4-2-1-3-5-18)27-28(29,25(20)26(22)35-27)14-15-31(23)17-19-6-7-19/h1-5,9-10,19,21,23,27,32,34H,6-8,11-17H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86932

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1cccc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:12:13:28.27.26:16.17.33,THB:14:13:28.27.26:16.17.33| Show InChI InChI=1S/C27H30N2O5/c30-18-3-1-2-17(12-18)25(32)28-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-29(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,30-31,33H,4-5,8-11,13-14H2,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86923
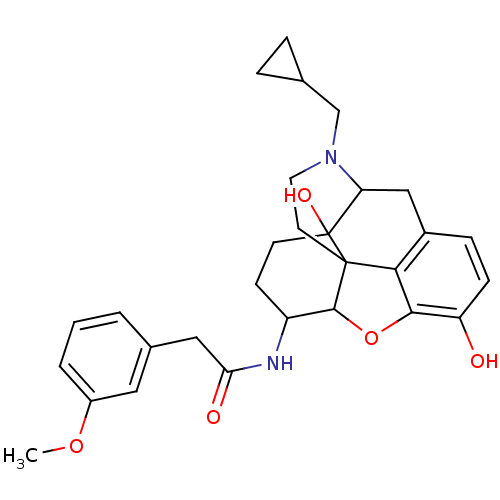
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cccc(CC(=O)NC2CCC3(O)C4Cc5ccc(O)c6OC2C3(CCN4CC2CC2)c56)c1 |TLB:15:14:17.18.34:27.28.29,13:14:17.18.34:27.28.29| Show InChI InChI=1S/C29H34N2O5/c1-35-20-4-2-3-18(13-20)14-24(33)30-21-9-10-29(34)23-15-19-7-8-22(32)26-25(19)28(29,27(21)36-26)11-12-31(23)16-17-5-6-17/h2-4,7-8,13,17,21,23,27,32,34H,5-6,9-12,14-16H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86929

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cccc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:15:14:17.18.34:27.28.29,13:14:17.18.34:27.28.29| Show InChI InChI=1S/C28H32N2O5/c1-34-19-4-2-3-18(13-19)26(32)29-20-9-10-28(33)22-14-17-7-8-21(31)24-23(17)27(28,25(20)35-24)11-12-30(22)15-16-5-6-16/h2-4,7-8,13,16,20,22,25,31,33H,5-6,9-12,14-15H2,1H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86935
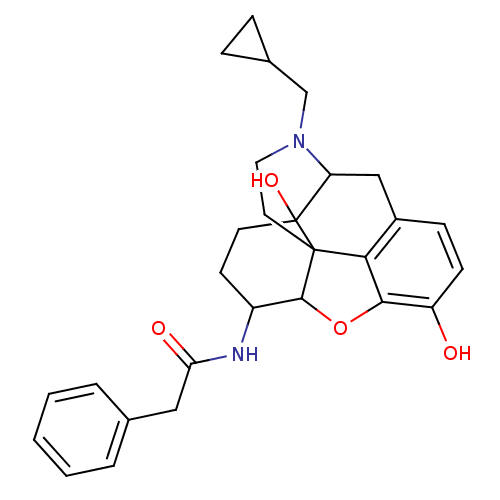
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)Cc1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C28H32N2O4/c31-21-9-8-19-15-22-28(33)11-10-20(29-23(32)14-17-4-2-1-3-5-17)26-27(28,24(19)25(21)34-26)12-13-30(22)16-18-6-7-18/h1-5,8-9,18,20,22,26,31,33H,6-7,10-16H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86941
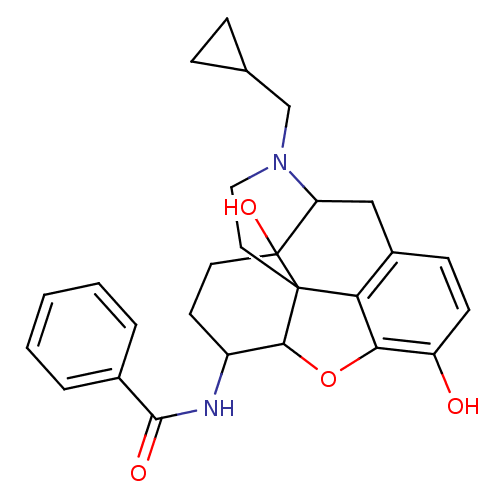
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H30N2O4/c30-20-9-8-18-14-21-27(32)11-10-19(28-25(31)17-4-2-1-3-5-17)24-26(27,22(18)23(20)33-24)12-13-29(21)15-16-6-7-16/h1-5,8-9,16,19,21,24,30,32H,6-7,10-15H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86941

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H30N2O4/c30-20-9-8-18-14-21-27(32)11-10-19(28-25(31)17-4-2-1-3-5-17)24-26(27,22(18)23(20)33-24)12-13-29(21)15-16-6-7-16/h1-5,8-9,16,19,21,24,30,32H,6-7,10-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86948
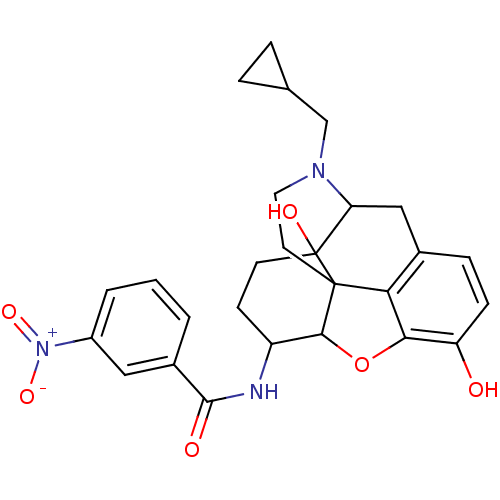
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1cccc(c1)[N+]([O-])=O |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H29N3O6/c31-20-7-6-16-13-21-27(33)9-8-19(28-25(32)17-2-1-3-18(12-17)30(34)35)24-26(27,22(16)23(20)36-24)10-11-29(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86926
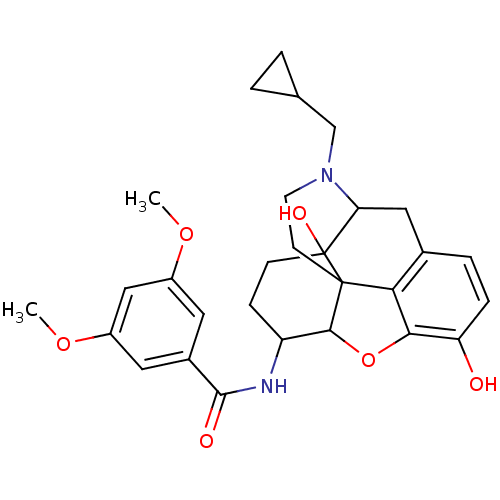
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cc(OC)cc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:17:16:19.20.36:29.30.31,15:16:19.20.36:29.30.31| Show InChI InChI=1S/C29H34N2O6/c1-35-19-11-18(12-20(14-19)36-2)27(33)30-21-7-8-29(34)23-13-17-5-6-22(32)25-24(17)28(29,26(21)37-25)9-10-31(23)15-16-3-4-16/h5-6,11-12,14,16,21,23,26,32,34H,3-4,7-10,13,15H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86947
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COC(=O)c1ccc(cc1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:17:16:19.20.36:29.30.31,15:16:19.20.36:29.30.31| Show InChI InChI=1S/C29H32N2O6/c1-36-27(34)18-6-4-17(5-7-18)26(33)30-20-10-11-29(35)22-14-19-8-9-21(32)24-23(19)28(29,25(20)37-24)12-13-31(22)15-16-2-3-16/h4-9,16,20,22,25,32,35H,2-3,10-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86941

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H30N2O4/c30-20-9-8-18-14-21-27(32)11-10-19(28-25(31)17-4-2-1-3-5-17)24-26(27,22(18)23(20)33-24)12-13-29(21)15-16-6-7-16/h1-5,8-9,16,19,21,24,30,32H,6-7,10-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304174
(4-chloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmeth...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H29ClN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86929

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cccc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:15:14:17.18.34:27.28.29,13:14:17.18.34:27.28.29| Show InChI InChI=1S/C28H32N2O5/c1-34-19-4-2-3-18(13-19)26(32)29-20-9-10-28(33)22-14-17-7-8-21(31)24-23(17)27(28,25(20)35-24)11-12-30(22)15-16-5-6-16/h2-4,7-8,13,16,20,22,25,31,33H,5-6,9-12,14-15H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86926

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cc(OC)cc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:17:16:19.20.36:29.30.31,15:16:19.20.36:29.30.31| Show InChI InChI=1S/C29H34N2O6/c1-35-19-11-18(12-20(14-19)36-2)27(33)30-21-7-8-29(34)23-13-17-5-6-22(32)25-24(17)28(29,26(21)37-25)9-10-31(23)15-16-3-4-16/h5-6,11-12,14,16,21,23,26,32,34H,3-4,7-10,13,15H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86945
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1ccc(CC(=O)NC2CCC3(O)C4Cc5ccc(O)c6OC2C3(CCN4CC2CC2)c56)cc1 |TLB:14:13:16.17.33:26.27.28,12:13:16.17.33:26.27.28| Show InChI InChI=1S/C29H34N2O5/c1-35-20-7-4-17(5-8-20)14-24(33)30-21-10-11-29(34)23-15-19-6-9-22(32)26-25(19)28(29,27(21)36-26)12-13-31(23)16-18-2-3-18/h4-9,18,21,23,27,32,34H,2-3,10-16H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86948

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1cccc(c1)[N+]([O-])=O |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H29N3O6/c31-20-7-6-16-13-21-27(33)9-8-19(28-25(32)17-2-1-3-18(12-17)30(34)35)24-26(27,22(16)23(20)36-24)10-11-29(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86926

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cc(OC)cc(c1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:17:16:19.20.36:29.30.31,15:16:19.20.36:29.30.31| Show InChI InChI=1S/C29H34N2O6/c1-35-19-11-18(12-20(14-19)36-2)27(33)30-21-7-8-29(34)23-13-17-5-6-22(32)25-24(17)28(29,26(21)37-25)9-10-31(23)15-16-3-4-16/h5-6,11-12,14,16,21,23,26,32,34H,3-4,7-10,13,15H2,1-2H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304170

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C27H29BrN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86493
(CAS_27943 | NALTREXONE-HCl | NSC_27943 | Naltrexon...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(=O)CCC35O |TLB:22:23:7.12.13:5.4.18,THB:24:23:7.12.13:5.4.18| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86946
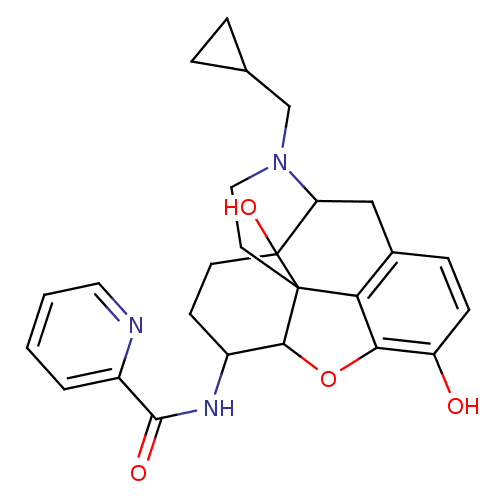
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccccn1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C26H29N3O4/c30-19-7-6-16-13-20-26(32)9-8-17(28-24(31)18-3-1-2-11-27-18)23-25(26,21(16)22(19)33-23)10-12-29(20)14-15-4-5-15/h1-3,6-7,11,15,17,20,23,30,32H,4-5,8-10,12-14H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50304175

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Cc1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C28H32N2O4/c1-16-2-6-18(7-3-16)26(32)29-20-10-11-28(33)22-14-19-8-9-21(31)24-23(19)27(28,25(20)34-24)12-13-30(22)15-17-4-5-17/h2-3,6-9,17,20,22,25,31,33H,4-5,10-15H2,1H3,(H,29,32)/t20-,22-,25+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304176
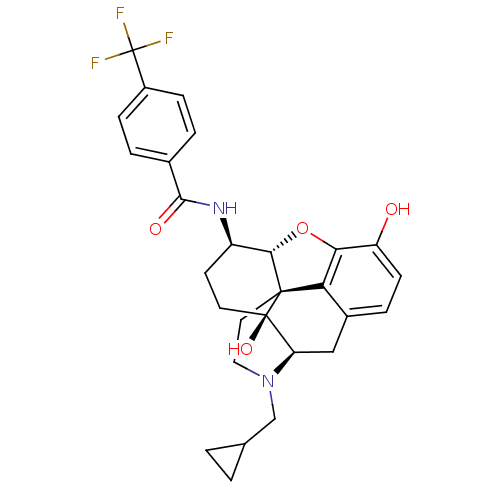
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C28H29F3N2O4/c29-28(30,31)18-6-3-16(4-7-18)25(35)32-19-9-10-27(36)21-13-17-5-8-20(34)23-22(17)26(27,24(19)37-23)11-12-33(21)14-15-1-2-15/h3-8,15,19,21,24,34,36H,1-2,9-14H2,(H,32,35)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304173
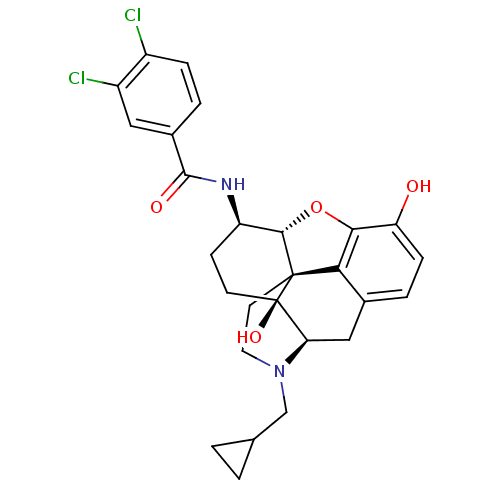
(3,4-dichloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropyl...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C27H28Cl2N2O4/c28-17-5-3-16(11-18(17)29)25(33)30-19-7-8-27(34)21-12-15-4-6-20(32)23-22(15)26(27,24(19)35-23)9-10-31(21)13-14-1-2-14/h3-6,11,14,19,21,24,32,34H,1-2,7-10,12-13H2,(H,30,33)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304172

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C31H38N2O4/c1-29(2,3)21-9-6-19(7-10-21)28(35)32-22-12-13-31(36)24-16-20-8-11-23(34)26-25(20)30(31,27(22)37-26)14-15-33(24)17-18-4-5-18/h6-11,18,22,24,27,34,36H,4-5,12-17H2,1-3H3,(H,32,35)/t22-,24-,27+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50304171
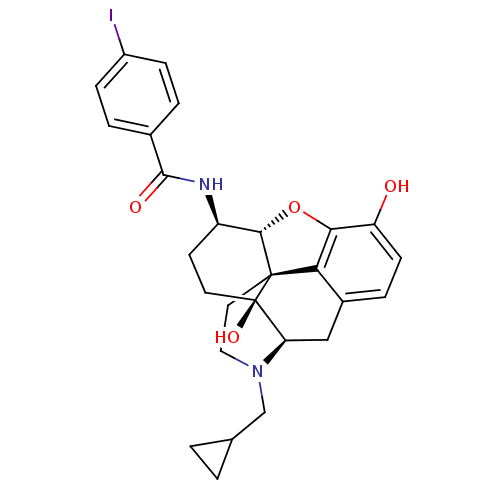
(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(I)cc1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86936

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccc(Cl)cc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C27H29ClN2O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86933
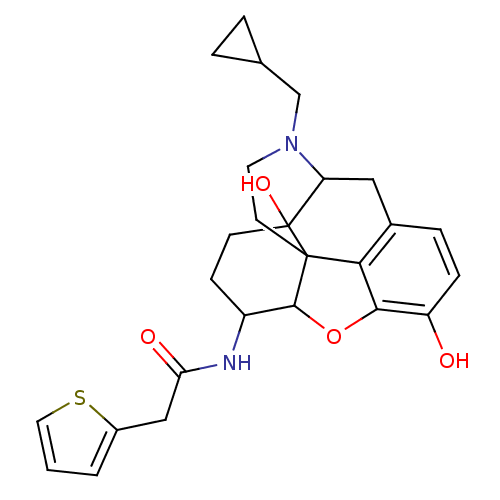
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)Cc1cccs1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C26H30N2O4S/c29-19-6-5-16-12-20-26(31)8-7-18(27-21(30)13-17-2-1-11-33-17)24-25(26,22(16)23(19)32-24)9-10-28(20)14-15-3-4-15/h1-2,5-6,11,15,18,20,24,29,31H,3-4,7-10,12-14H2,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86946

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)c1ccccn1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C26H29N3O4/c30-19-7-6-16-13-20-26(32)9-8-17(28-24(31)18-3-1-2-11-27-18)23-25(26,21(16)22(19)33-23)10-12-29(20)14-15-4-5-15/h1-3,6-7,11,15,17,20,23,30,32H,4-5,8-10,12-14H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86938
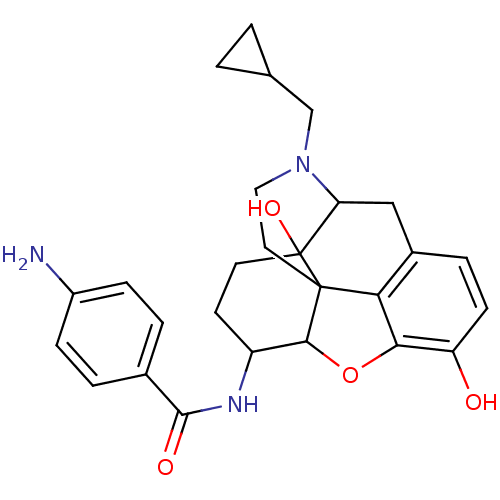
(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Nc1ccc(cc1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:12:13:28.27.26:16.17.33,THB:14:13:28.27.26:16.17.33| Show InChI InChI=1S/C27H31N3O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14,28H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86945

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1ccc(CC(=O)NC2CCC3(O)C4Cc5ccc(O)c6OC2C3(CCN4CC2CC2)c56)cc1 |TLB:14:13:16.17.33:26.27.28,12:13:16.17.33:26.27.28| Show InChI InChI=1S/C29H34N2O5/c1-35-20-7-4-17(5-8-20)14-24(33)30-21-10-11-29(34)23-15-19-6-9-22(32)26-25(19)28(29,27(21)36-26)12-13-31(23)16-18-2-3-18/h4-9,18,21,23,27,32,34H,2-3,10-16H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86938

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Nc1ccc(cc1)C(=O)NC1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45 |TLB:12:13:28.27.26:16.17.33,THB:14:13:28.27.26:16.17.33| Show InChI InChI=1S/C27H31N3O4/c28-18-6-3-16(4-7-18)25(32)29-19-9-10-27(33)21-13-17-5-8-20(31)23-22(17)26(27,24(19)34-23)11-12-30(21)14-15-1-2-15/h3-8,15,19,21,24,31,33H,1-2,9-14,28H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86933

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)Cc1cccs1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C26H30N2O4S/c29-19-6-5-16-12-20-26(31)8-7-18(27-21(30)13-17-2-1-11-33-17)24-25(26,22(16)23(19)32-24)9-10-28(20)14-15-3-4-15/h1-2,5-6,11,15,18,20,24,29,31H,3-4,7-10,12-14H2,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86935

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)NC(=O)Cc1ccccc1 |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C28H32N2O4/c31-21-9-8-19-15-22-28(33)11-10-20(29-23(32)14-17-4-2-1-3-5-17)26-27(28,24(19)25(21)34-26)12-13-30(22)16-18-6-7-18/h1-5,8-9,18,20,22,26,31,33H,6-7,10-16H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86923

(17-cyclopropylmethyl-3,14-beta-dihydroxy-4,5-alpha...)Show SMILES COc1cccc(CC(=O)NC2CCC3(O)C4Cc5ccc(O)c6OC2C3(CCN4CC2CC2)c56)c1 |TLB:15:14:17.18.34:27.28.29,13:14:17.18.34:27.28.29| Show InChI InChI=1S/C29H34N2O5/c1-35-20-4-2-3-18(13-20)14-24(33)30-21-9-10-29(34)23-15-19-7-8-22(32)26-25(19)28(29,27(21)36-26)11-12-31(23)16-17-5-6-17/h2-4,7-8,13,17,21,23,27,32,34H,5-6,9-12,14-16H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by PDSP Ki Database
| |
J Med Chem 51: 1913-24 (2008)
Article DOI: 10.1021/jm701060e
BindingDB Entry DOI: 10.7270/Q2KS6Q4K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50304176

(17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C28H29F3N2O4/c29-28(30,31)18-6-3-16(4-7-18)25(35)32-19-9-10-27(36)21-13-17-5-8-20(34)23-22(17)26(27,24(19)37-23)11-12-33(21)14-15-1-2-15/h3-8,15,19,21,24,34,36H,1-2,9-14H2,(H,32,35)/t19-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data