 Found 100 hits with Last Name = 'ghodsi' and Initial = 'r'
Found 100 hits with Last Name = 'ghodsi' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50442322

(CHEMBL2442760)Show InChI InChI=1S/C23H20N4/c1-2-27-12-10-19-22(27)9-8-20-23(19)18(15-26-13-11-24-16-26)14-21(25-20)17-6-4-3-5-7-17/h3-14,16H,2,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442326

(CHEMBL2442758)Show InChI InChI=1S/C23H20N4/c1-2-27-12-10-20-22(27)9-8-19-18(15-26-13-11-24-16-26)14-21(25-23(19)20)17-6-4-3-5-7-17/h3-14,16H,2,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442323
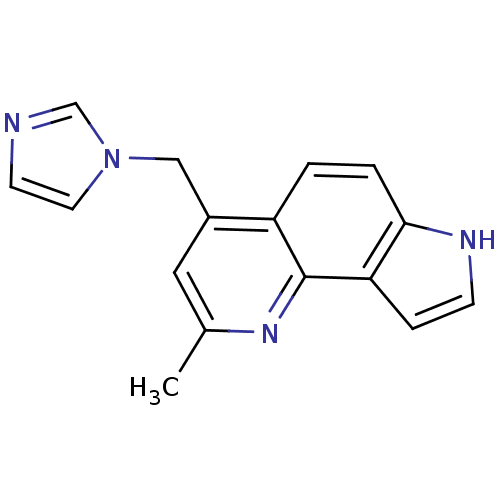
(CHEMBL2442759)Show InChI InChI=1S/C16H14N4/c1-11-8-12(9-20-7-6-17-10-20)13-2-3-15-14(4-5-18-15)16(13)19-11/h2-8,10,18H,9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442325
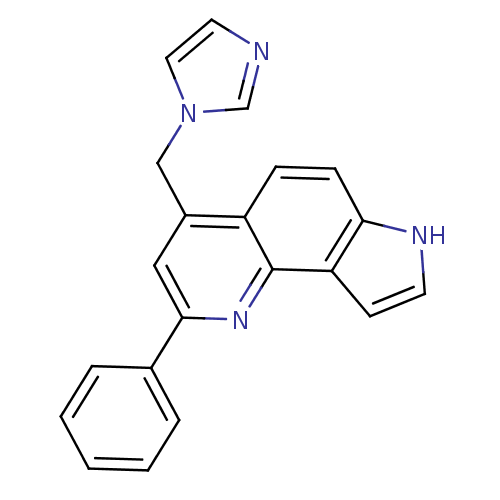
(CHEMBL2442756)Show SMILES C(c1cc(nc2c3cc[nH]c3ccc12)-c1ccccc1)n1ccnc1 Show InChI InChI=1S/C21H16N4/c1-2-4-15(5-3-1)20-12-16(13-25-11-10-22-14-25)17-6-7-19-18(8-9-23-19)21(17)24-20/h1-12,14,23H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442320
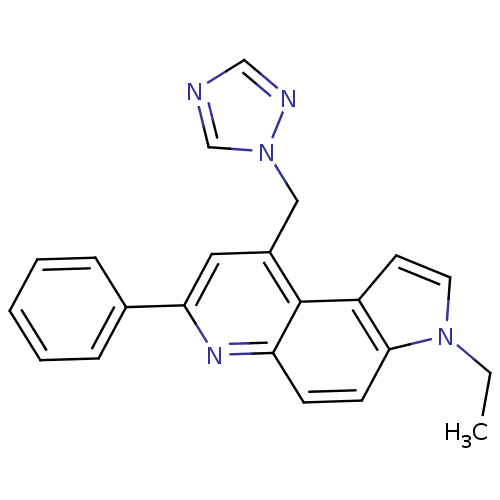
(CHEMBL2442762)Show InChI InChI=1S/C22H19N5/c1-2-26-11-10-18-21(26)9-8-19-22(18)17(13-27-15-23-14-24-27)12-20(25-19)16-6-4-3-5-7-16/h3-12,14-15H,2,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300865

(7,8,9,10-Tetrahydro-2-(4-(methylsulfonyl)phenyl)be...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2ccc3CCCCc3c2n1 Show InChI InChI=1S/C21H19NO4S/c1-27(25,26)15-9-6-14(7-10-15)19-12-18(21(23)24)17-11-8-13-4-2-3-5-16(13)20(17)22-19/h6-12H,2-5H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300866
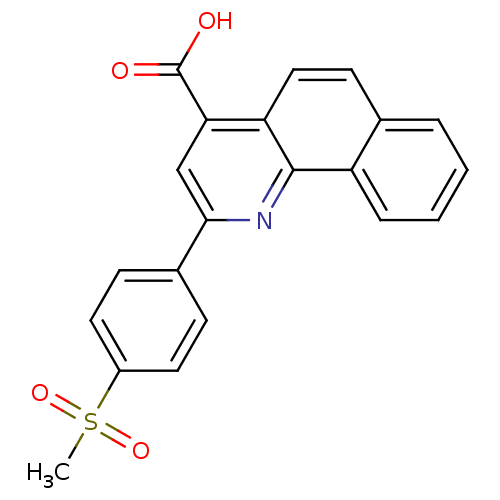
(2-(4-(Methylsulfonyl)phenyl)benzo[h]quinoline-4-ca...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2ccc3ccccc3c2n1 Show InChI InChI=1S/C21H15NO4S/c1-27(25,26)15-9-6-14(7-10-15)19-12-18(21(23)24)17-11-8-13-4-2-3-5-16(13)20(17)22-19/h2-12H,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326830
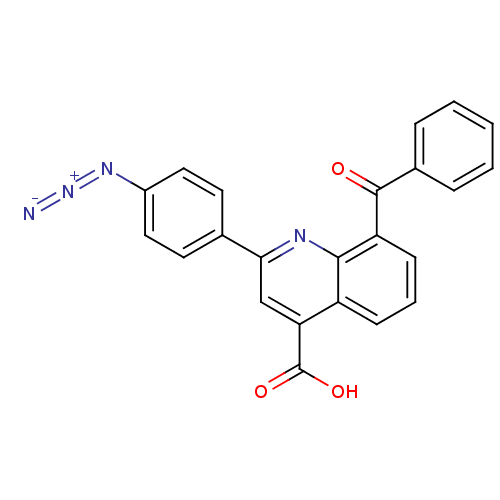
(2-(4-azido)phenyl)-8-benzoyl-quinoline-4-carboxyli...)Show SMILES OC(=O)c1cc(nc2c(cccc12)C(=O)c1ccccc1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C23H14N4O3/c24-27-26-16-11-9-14(10-12-16)20-13-19(23(29)30)17-7-4-8-18(21(17)25-20)22(28)15-5-2-1-3-6-15/h1-13H,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326828

(8-benzoyl-2-(4-(methylsulfonyl)phenyl)quinoline-4-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2cccc(C(=O)c3ccccc3)c2n1 Show InChI InChI=1S/C24H17NO5S/c1-31(29,30)17-12-10-15(11-13-17)21-14-20(24(27)28)18-8-5-9-19(22(18)25-21)23(26)16-6-3-2-4-7-16/h2-14H,1H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50306357
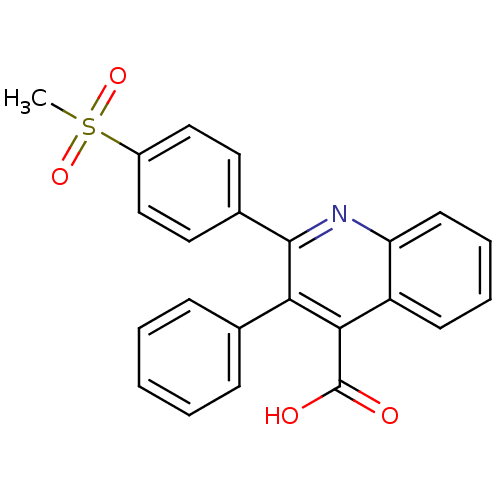
(2-(4-(methylsulfonyl)phenyl)-3-phenylquinoline-4-c...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc2ccccc2c(C(O)=O)c1-c1ccccc1 Show InChI InChI=1S/C23H17NO4S/c1-29(27,28)17-13-11-16(12-14-17)22-20(15-7-3-2-4-8-15)21(23(25)26)18-9-5-6-10-19(18)24-22/h2-14H,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300867
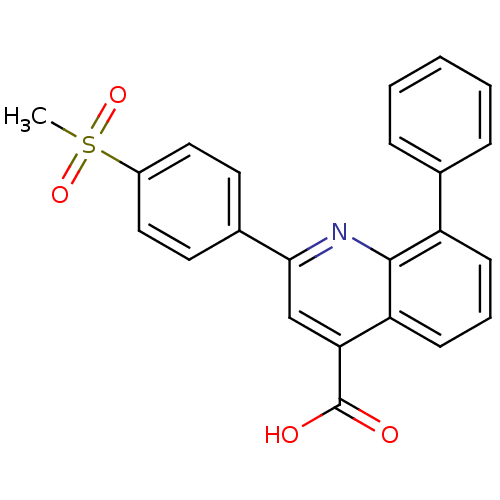
(2-(4-(Methylsulfonyl)phenyl)-8-phenyl-quinoline-4-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2cccc(-c3ccccc3)c2n1 Show InChI InChI=1S/C23H17NO4S/c1-29(27,28)17-12-10-16(11-13-17)21-14-20(23(25)26)19-9-5-8-18(22(19)24-21)15-6-3-2-4-7-15/h2-14H,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300868
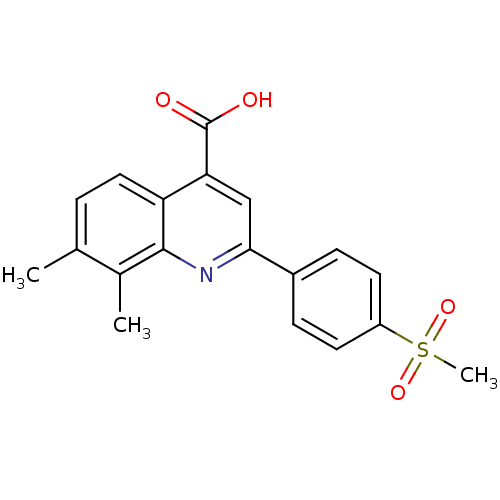
(7,8-Dimethyl-2-(4-(methylsulfonyl)phenyl)quinoline...)Show SMILES Cc1ccc2c(cc(nc2c1C)-c1ccc(cc1)S(C)(=O)=O)C(O)=O Show InChI InChI=1S/C19H17NO4S/c1-11-4-9-15-16(19(21)22)10-17(20-18(15)12(11)2)13-5-7-14(8-6-13)25(3,23)24/h4-10H,1-3H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50442323

(CHEMBL2442759)Show InChI InChI=1S/C16H14N4/c1-11-8-12(9-20-7-6-17-10-20)13-2-3-15-14(4-5-18-15)16(13)19-11/h2-8,10,18H,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326831
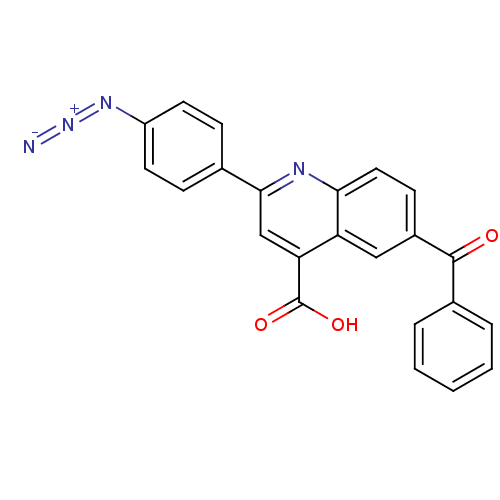
(2-(4-azido)phenyl)-6-benzoyl-quinoline-4-carboxyli...)Show SMILES OC(=O)c1cc(nc2ccc(cc12)C(=O)c1ccccc1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C23H14N4O3/c24-27-26-17-9-6-14(7-10-17)21-13-19(23(29)30)18-12-16(8-11-20(18)25-21)22(28)15-4-2-1-3-5-15/h1-13H,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326829

(6-benzoyl-2-(4-(methylsulfonyl)phenyl)quinoline-4-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2cc(ccc2n1)C(=O)c1ccccc1 Show InChI InChI=1S/C24H17NO5S/c1-31(29,30)18-10-7-15(8-11-18)22-14-20(24(27)28)19-13-17(9-12-21(19)25-22)23(26)16-5-3-2-4-6-16/h2-14H,1H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50306356

(4-amino-2-(4-(methylsulfonyl)phenyl)-3-phenylquino...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc2ccccc2c(N)c1-c1ccccc1 Show InChI InChI=1S/C22H18N2O2S/c1-27(25,26)17-13-11-16(12-14-17)22-20(15-7-3-2-4-8-15)21(23)18-9-5-6-10-19(18)24-22/h2-14H,1H3,(H2,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326826
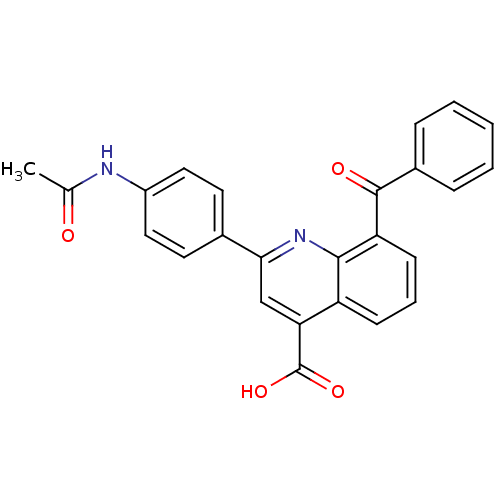
(2-(4-acetamido)phenyl)-8-benzoyl-quinoline-4-carbo...)Show SMILES CC(=O)Nc1ccc(cc1)-c1cc(C(O)=O)c2cccc(C(=O)c3ccccc3)c2n1 Show InChI InChI=1S/C25H18N2O4/c1-15(28)26-18-12-10-16(11-13-18)22-14-21(25(30)31)19-8-5-9-20(23(19)27-22)24(29)17-6-3-2-4-7-17/h2-14H,1H3,(H,26,28)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300864
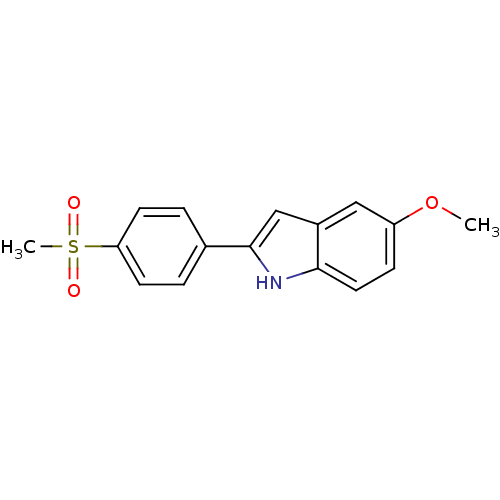
(5-methoxy-2-(4-(methylsulfonyl)phenyl)-1H-indole |...)Show InChI InChI=1S/C16H15NO3S/c1-20-13-5-8-15-12(9-13)10-16(17-15)11-3-6-14(7-4-11)21(2,18)19/h3-10,17H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50326827
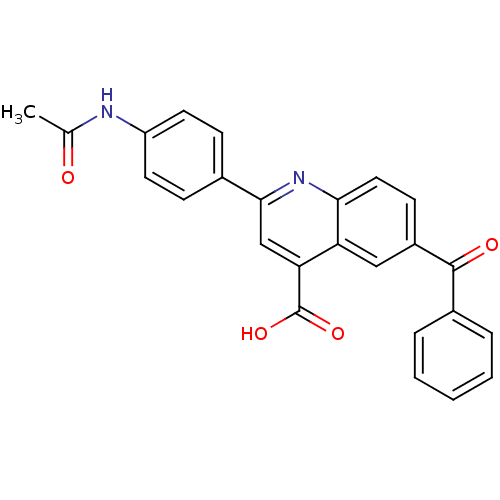
(2-(4-acetamido)phenyl)-6-benzoyl-quinoline-4-carbo...)Show SMILES CC(=O)Nc1ccc(cc1)-c1cc(C(O)=O)c2cc(ccc2n1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18N2O4/c1-15(28)26-19-10-7-16(8-11-19)23-14-21(25(30)31)20-13-18(9-12-22(20)27-23)24(29)17-5-3-2-4-6-17/h2-14H,1H3,(H,26,28)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300869

(8-Methyl-2-(4-(methylsulfonyl)phenyl)quinoline-4-c...)Show SMILES Cc1cccc2c(cc(nc12)-c1ccc(cc1)S(C)(=O)=O)C(O)=O Show InChI InChI=1S/C18H15NO4S/c1-11-4-3-5-14-15(18(20)21)10-16(19-17(11)14)12-6-8-13(9-7-12)24(2,22)23/h3-10H,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Ovis aries) | BDBM50300870

(2-(4-(methylsulfonyl)phenyl)-quinoline-4-carboxyli...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2ccccc2n1 Show InChI InChI=1S/C17H13NO4S/c1-23(21,22)12-8-6-11(7-9-12)16-10-14(17(19)20)13-4-2-3-5-15(13)18-16/h2-10H,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 |
Bioorg Med Chem 18: 5855-60 (2010)
Article DOI: 10.1016/j.bmc.2010.06.094
BindingDB Entry DOI: 10.7270/Q23J3D6V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50300870

(2-(4-(methylsulfonyl)phenyl)-quinoline-4-carboxyli...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(C(O)=O)c2ccccc2n1 Show InChI InChI=1S/C17H13NO4S/c1-23(21,22)12-8-6-11(7-9-12)16-10-14(17(19)20)13-4-2-3-5-15(13)18-16/h2-10H,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University (M.C)
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by chemiluminescent enzyme assay |
Bioorg Med Chem 17: 5312-7 (2009)
Article DOI: 10.1016/j.bmc.2009.05.084
BindingDB Entry DOI: 10.7270/Q2NS0TZX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50306353
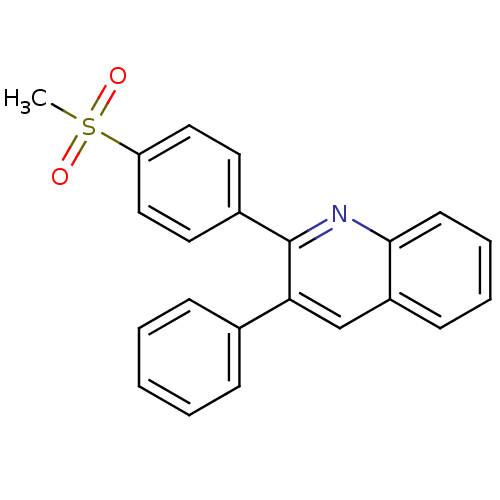
(2-(4-(methylsulfonyl)phenyl)-3-phenylquinoline | C...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc2ccccc2cc1-c1ccccc1 Show InChI InChI=1S/C22H17NO2S/c1-26(24,25)19-13-11-17(12-14-19)22-20(16-7-3-2-4-8-16)15-18-9-5-6-10-21(18)23-22/h2-15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50306354
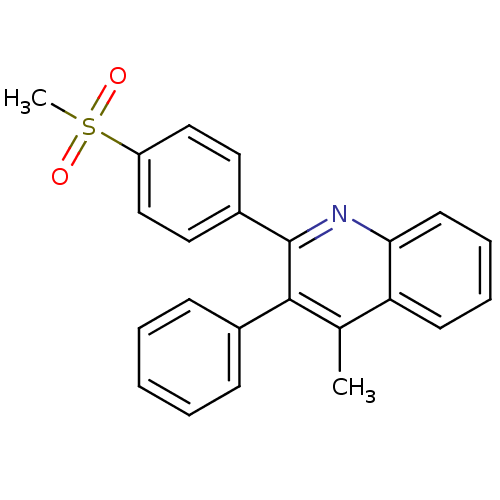
(4-methyl-2-(4-methylsulfonyl)phenyl)-3-phenylquino...)Show SMILES Cc1c(-c2ccccc2)c(nc2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C23H19NO2S/c1-16-20-10-6-7-11-21(20)24-23(22(16)17-8-4-3-5-9-17)18-12-14-19(15-13-18)27(2,25)26/h3-15H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50306355
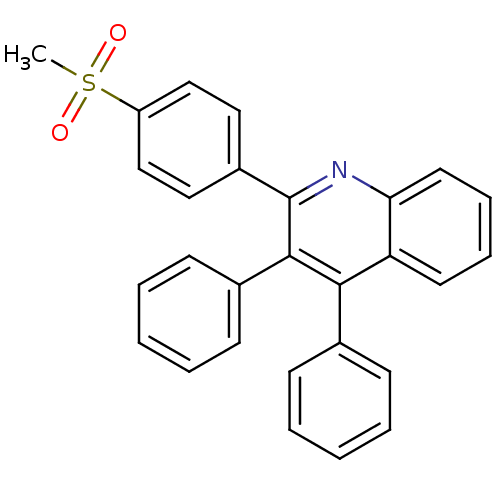
(3,4-diphenyl-2-(4-methylsulfonyl)phenyl)-quinoline...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc2ccccc2c(-c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C28H21NO2S/c1-32(30,31)23-18-16-22(17-19-23)28-27(21-12-6-3-7-13-21)26(20-10-4-2-5-11-20)24-14-8-9-15-25(24)29-28/h2-19H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shahid Beheshti University MC
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme chemiluminescent enzyme assay |
Bioorg Med Chem 18: 1029-33 (2010)
Article DOI: 10.1016/j.bmc.2009.12.060
BindingDB Entry DOI: 10.7270/Q2V40V94 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50442325

(CHEMBL2442756)Show SMILES C(c1cc(nc2c3cc[nH]c3ccc12)-c1ccccc1)n1ccnc1 Show InChI InChI=1S/C21H16N4/c1-2-4-15(5-3-1)20-12-16(13-25-11-10-22-14-25)17-6-7-19-18(8-9-23-19)21(17)24-20/h1-12,14,23H,13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50442326

(CHEMBL2442758)Show InChI InChI=1S/C23H20N4/c1-2-27-12-10-20-22(27)9-8-19-18(15-26-13-11-24-16-26)14-21(25-23(19)20)17-6-4-3-5-7-17/h3-14,16H,2,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50464816

(CHEMBL4285001)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccc(OCCOc3no[n+]([O-])c3S(=O)(=O)c3ccccc3)nc2)cc1 Show InChI InChI=1S/C31H28N6O9S/c32-25-8-4-5-9-26(25)35-28(38)23-13-10-21(11-14-23)18-34-31(39)45-20-22-12-15-27(33-19-22)43-16-17-44-29-30(37(40)46-36-29)47(41,42)24-6-2-1-3-7-24/h1-15,19H,16-18,20,32H2,(H,34,39)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50597696
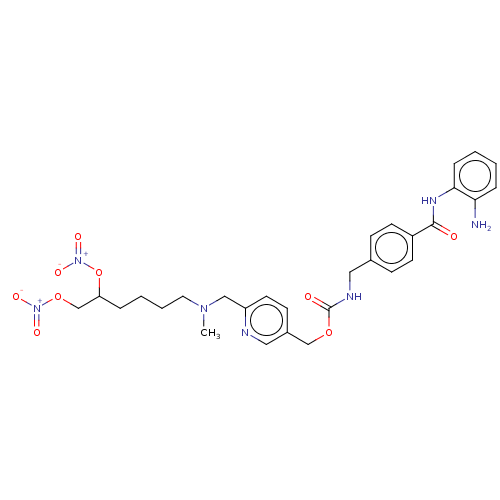
(CHEMBL4300553)Show SMILES CN(CCCCC(CO[N+]([O-])=O)O[N+]([O-])=O)Cc1ccc(COC(=O)NCc2ccc(cc2)C(=O)Nc2ccccc2N)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using deacetylase fluorogenic substrate pretreated for 5 mins followed by substrate addition after 30 mins by fl... |
Eur J Med Chem 132: 42-62 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.024
BindingDB Entry DOI: 10.7270/Q2NC63MW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442324
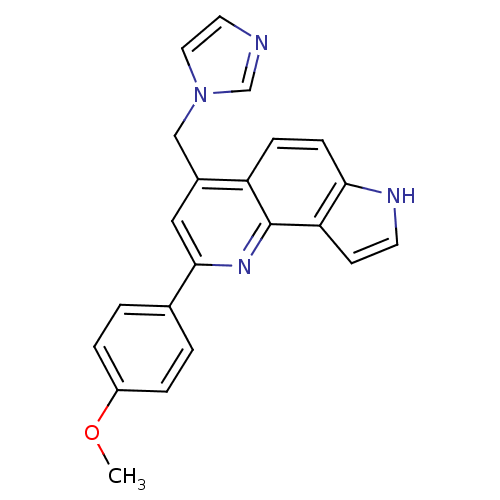
(CHEMBL2442757)Show SMILES COc1ccc(cc1)-c1cc(Cn2ccnc2)c2ccc3[nH]ccc3c2n1 Show InChI InChI=1S/C22H18N4O/c1-27-17-4-2-15(3-5-17)21-12-16(13-26-11-10-23-14-26)18-6-7-20-19(8-9-24-20)22(18)25-21/h2-12,14,24H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 454 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50248809
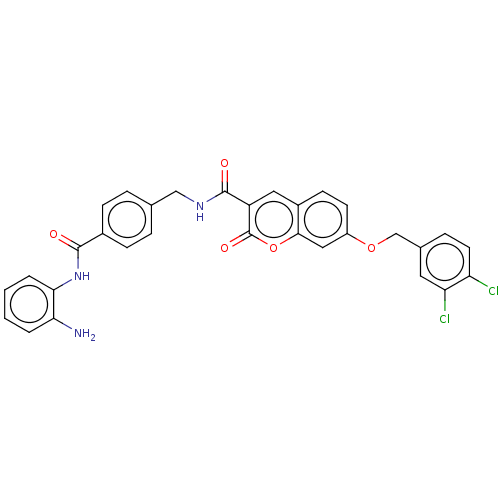
(CHEMBL4078308)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)c2cc3ccc(OCc4ccc(Cl)c(Cl)c4)cc3oc2=O)cc1 Show InChI InChI=1S/C31H23Cl2N3O5/c32-24-12-7-19(13-25(24)33)17-40-22-11-10-21-14-23(31(39)41-28(21)15-22)30(38)35-16-18-5-8-20(9-6-18)29(37)36-27-4-2-1-3-26(27)34/h1-15H,16-17,34H2,(H,35,38)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using deacetylase fluorogenic substrate pretreated for 5 mins followed by substrate addition after 30 mins by fl... |
Eur J Med Chem 132: 42-62 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.024
BindingDB Entry DOI: 10.7270/Q2NC63MW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50442326

(CHEMBL2442758)Show InChI InChI=1S/C23H20N4/c1-2-27-12-10-20-22(27)9-8-19-18(15-26-13-11-24-16-26)14-21(25-23(19)20)17-6-4-3-5-7-17/h3-14,16H,2,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50248808
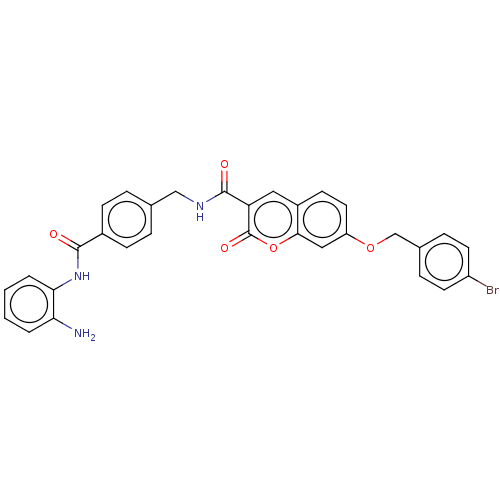
(CHEMBL4069655)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)c2cc3ccc(OCc4ccc(Br)cc4)cc3oc2=O)cc1 Show InChI InChI=1S/C31H24BrN3O5/c32-23-12-7-20(8-13-23)18-39-24-14-11-22-15-25(31(38)40-28(22)16-24)30(37)34-17-19-5-9-21(10-6-19)29(36)35-27-4-2-1-3-26(27)33/h1-16H,17-18,33H2,(H,34,37)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using deacetylase fluorogenic substrate pretreated for 5 mins followed by substrate addition after 30 mins by fl... |
Eur J Med Chem 132: 42-62 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.024
BindingDB Entry DOI: 10.7270/Q2NC63MW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50442324

(CHEMBL2442757)Show SMILES COc1ccc(cc1)-c1cc(Cn2ccnc2)c2ccc3[nH]ccc3c2n1 Show InChI InChI=1S/C22H18N4O/c1-27-17-4-2-15(3-5-17)21-12-16(13-26-11-10-23-14-26)18-6-7-20-19(8-9-24-20)22(18)25-21/h2-12,14,24H,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442316
(CHEMBL2442766)Show SMILES COc1cccc(c1)C1=CC(=O)c2ccc3N=CC=c3c2=N1 |c:17,19,23,t:9| Show InChI InChI=1S/C18H12N2O2/c1-22-12-4-2-3-11(9-12)16-10-17(21)14-5-6-15-13(7-8-19-15)18(14)20-16/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50248810
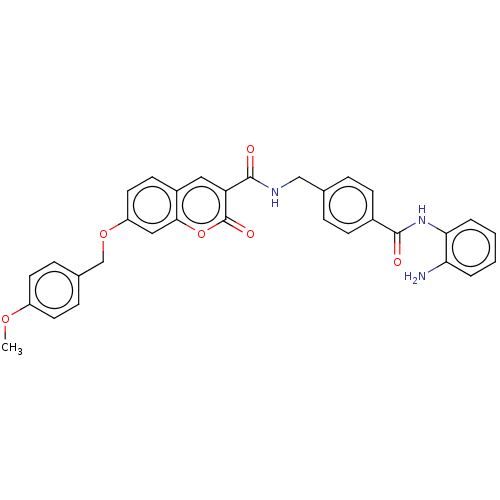
(CHEMBL4102954)Show SMILES COc1ccc(COc2ccc3cc(C(=O)NCc4ccc(cc4)C(=O)Nc4ccccc4N)c(=O)oc3c2)cc1 Show InChI InChI=1S/C32H27N3O6/c1-39-24-13-8-21(9-14-24)19-40-25-15-12-23-16-26(32(38)41-29(23)17-25)31(37)34-18-20-6-10-22(11-7-20)30(36)35-28-5-3-2-4-27(28)33/h2-17H,18-19,33H2,1H3,(H,34,37)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using deacetylase fluorogenic substrate pretreated for 5 mins followed by substrate addition after 30 mins by fl... |
Eur J Med Chem 132: 42-62 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.024
BindingDB Entry DOI: 10.7270/Q2NC63MW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50248807
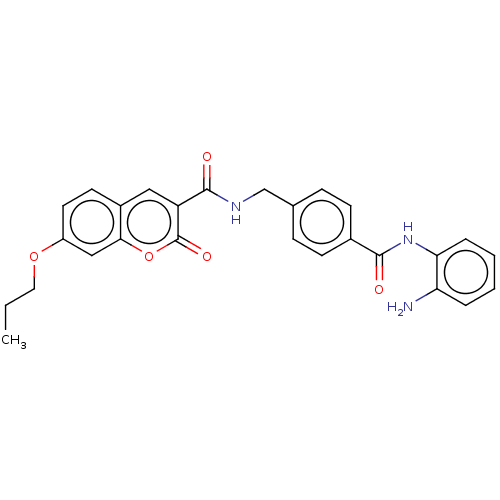
(CHEMBL4087376)Show SMILES CCCOc1ccc2cc(C(=O)NCc3ccc(cc3)C(=O)Nc3ccccc3N)c(=O)oc2c1 Show InChI InChI=1S/C27H25N3O5/c1-2-13-34-20-12-11-19-14-21(27(33)35-24(19)15-20)26(32)29-16-17-7-9-18(10-8-17)25(31)30-23-6-4-3-5-22(23)28/h3-12,14-15H,2,13,16,28H2,1H3,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using deacetylase fluorogenic substrate pretreated for 5 mins followed by substrate addition after 30 mins by fl... |
Eur J Med Chem 132: 42-62 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.024
BindingDB Entry DOI: 10.7270/Q2NC63MW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50442325

(CHEMBL2442756)Show SMILES C(c1cc(nc2c3cc[nH]c3ccc12)-c1ccccc1)n1ccnc1 Show InChI InChI=1S/C21H16N4/c1-2-4-15(5-3-1)20-12-16(13-25-11-10-22-14-25)17-6-7-19-18(8-9-23-19)21(17)24-20/h1-12,14,23H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli using progesterone as substrate NADPH as cofactor |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50442330

(CHEMBL2440145)Show InChI InChI=1S/C19H16N2O2/c1-23-14-4-2-12(3-5-14)18-10-13(11-22)15-6-7-17-16(8-9-20-17)19(15)21-18/h2-10,20,22H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 922 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) using 7-methoxy-4-trifluoromethylcoumarin as substrate after 30 mins by fluorimetric analysis |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50442324

(CHEMBL2442757)Show SMILES COc1ccc(cc1)-c1cc(Cn2ccnc2)c2ccc3[nH]ccc3c2n1 Show InChI InChI=1S/C22H18N4O/c1-27-17-4-2-15(3-5-17)21-12-16(13-26-11-10-23-14-26)18-6-7-20-19(8-9-24-20)22(18)25-21/h2-12,14,24H,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50442322

(CHEMBL2442760)Show InChI InChI=1S/C23H20N4/c1-2-27-12-10-19-22(27)9-8-20-23(19)18(15-26-13-11-24-16-26)14-21(25-20)17-6-4-3-5-7-17/h3-14,16H,2,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 using [1,2-3H]-11-deoxycorticosterone as substrate |
J Med Chem 56: 7536-51 (2013)
Article DOI: 10.1021/jm400377z
BindingDB Entry DOI: 10.7270/Q2M046WV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50464816

(CHEMBL4285001)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccc(OCCOc3no[n+]([O-])c3S(=O)(=O)c3ccccc3)nc2)cc1 Show InChI InChI=1S/C31H28N6O9S/c32-25-8-4-5-9-26(25)35-28(38)23-13-10-21(11-14-23)18-34-31(39)45-20-22-12-15-27(33-19-22)43-16-17-44-29-30(37(40)46-36-29)47(41,42)24-6-2-1-3-7-24/h1-15,19H,16-18,20,32H2,(H,34,39)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50597696

(CHEMBL4300553)Show SMILES CN(CCCCC(CO[N+]([O-])=O)O[N+]([O-])=O)Cc1ccc(COC(=O)NCc2ccc(cc2)C(=O)Nc2ccccc2N)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113934
BindingDB Entry DOI: 10.7270/Q2QV3RJ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data