

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50447027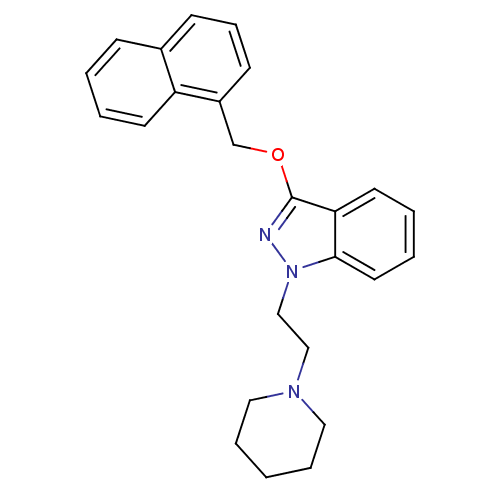 (CHEMBL3116284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447026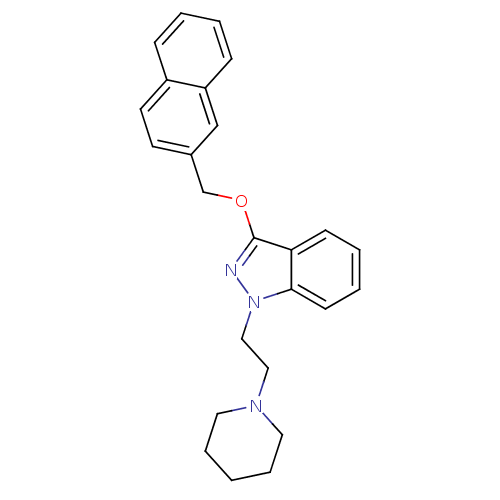 (CHEMBL3116286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447015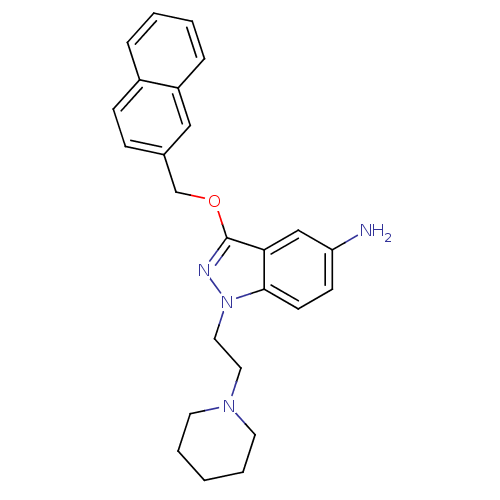 (CHEMBL3116300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447020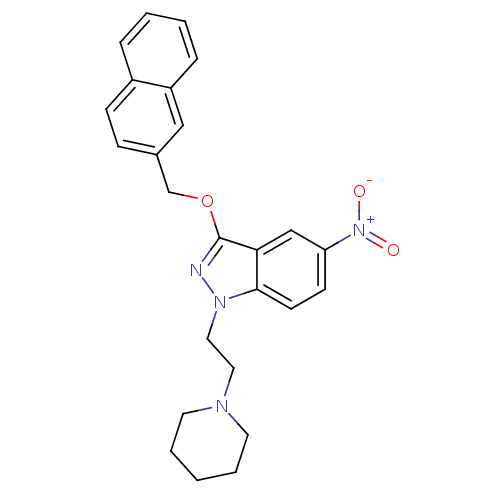 (CHEMBL3116294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447030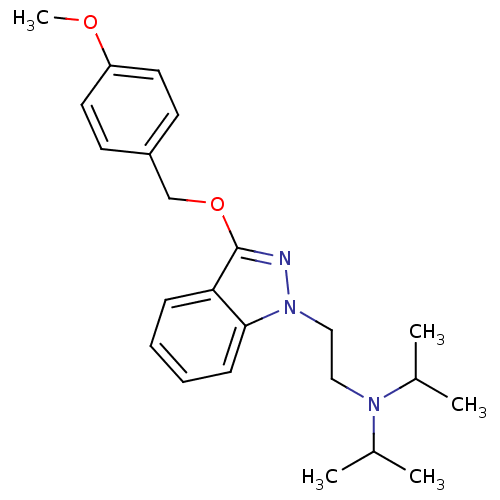 (CHEMBL3116280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447028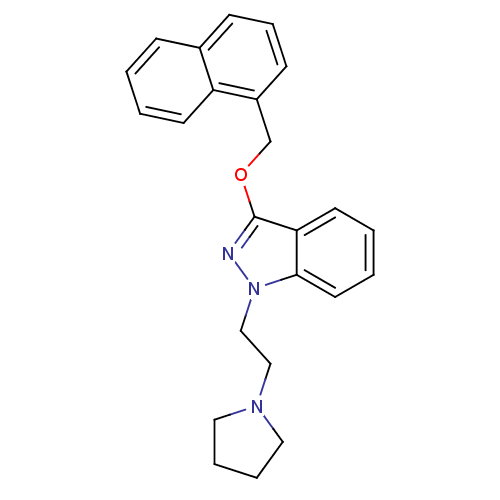 (CHEMBL3116283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447018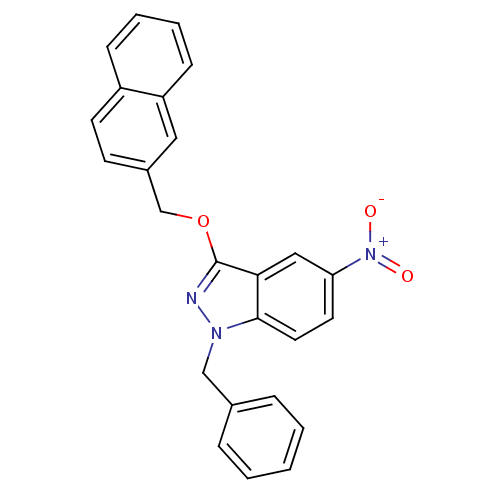 (CHEMBL3116296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447029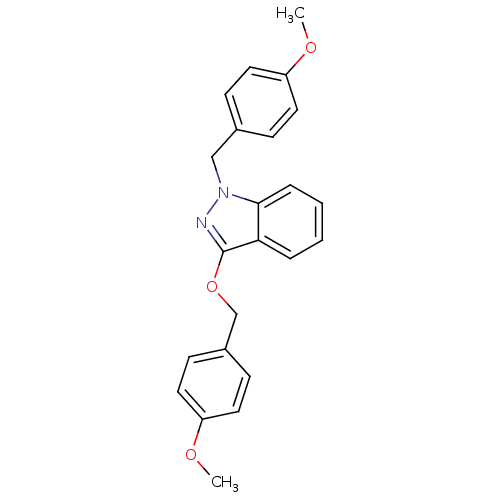 (CHEMBL3116281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447025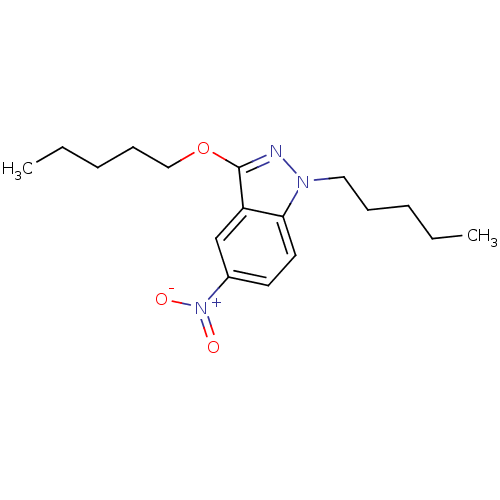 (CHEMBL3116287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447024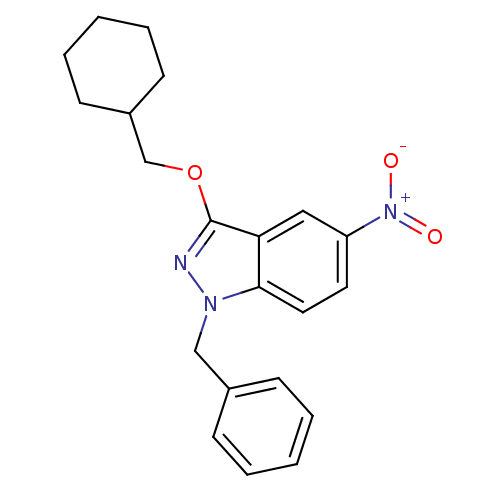 (CHEMBL3116288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447023 (CHEMBL1973869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447022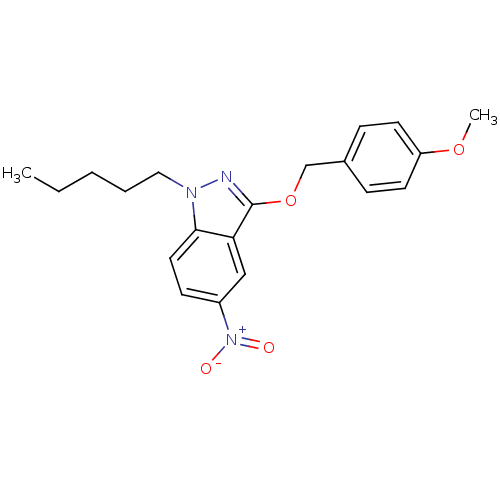 (CHEMBL3116289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447021 (CHEMBL3116293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447019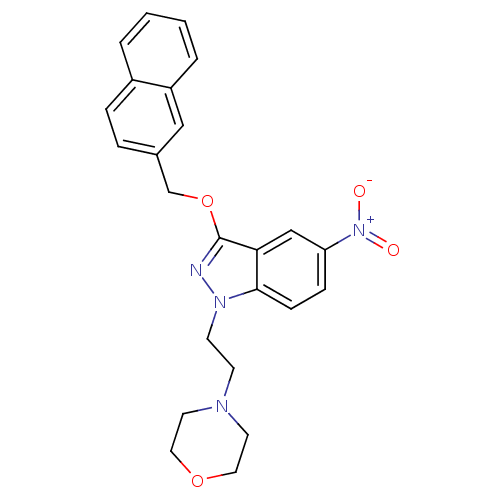 (CHEMBL3116295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447031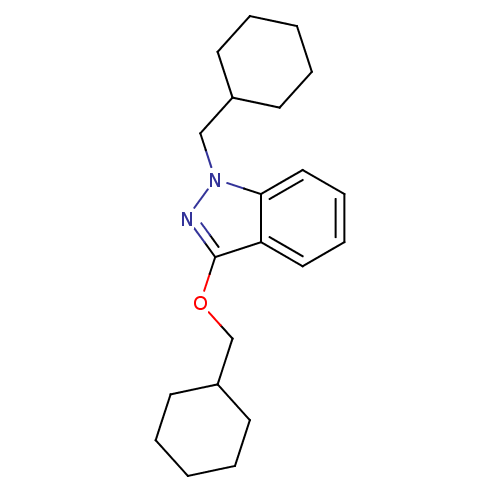 (CHEMBL3116278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447016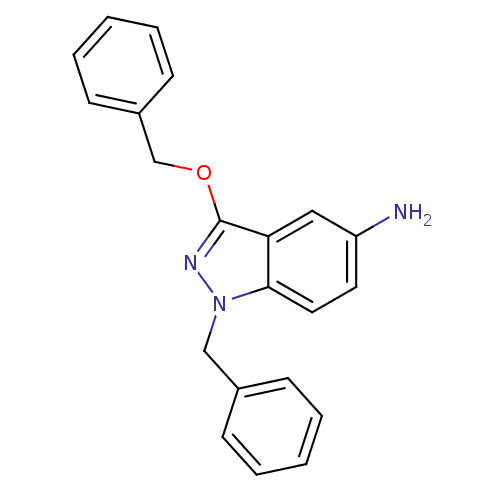 (CHEMBL3116298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447017 (CHEMBL3116297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292327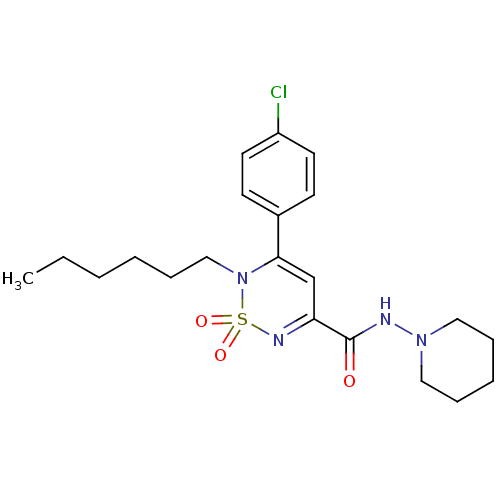 (CHEMBL241774 | N-(piperidin-1-yl)-3-(4-chloropheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.44E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292325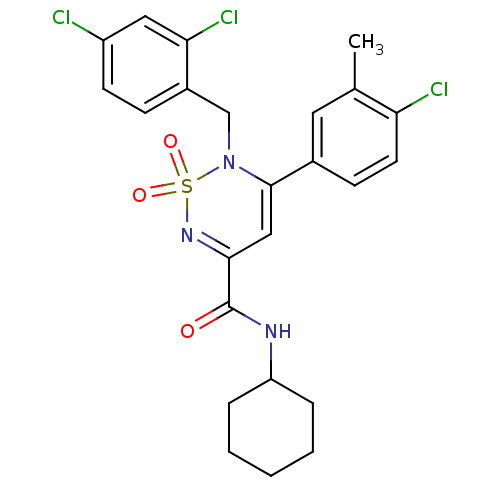 (CHEMBL240109 | N-cyclohexyl-2-(2,4-dichlorobenzyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292326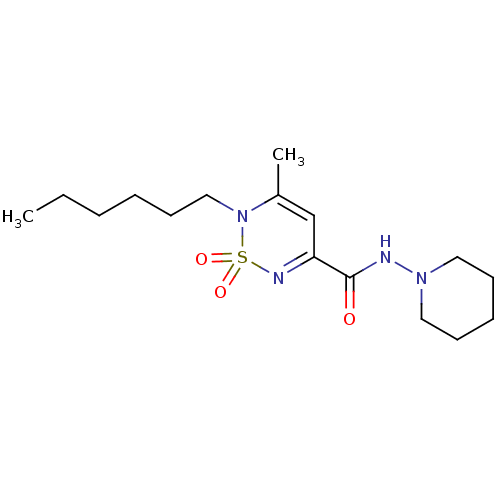 (CHEMBL239257 | N-(piperidin-1-yl)-2-hexyl-3-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292329 (CHEMBL240324 | N-(piperidin-1-yl)-2-benzyl-3-(4-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292328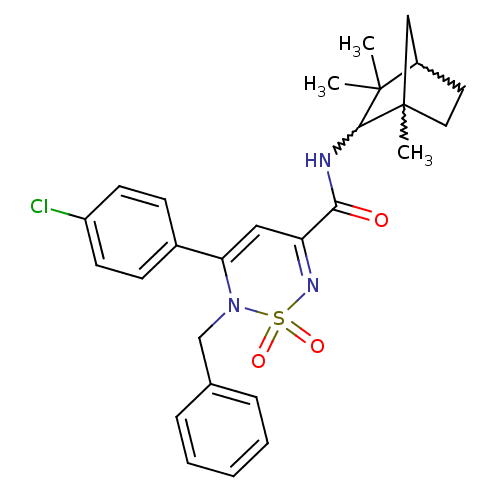 (CHEMBL241567 | N-(1,3,3-trimethylbicyclo[2.2.1]hep...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292330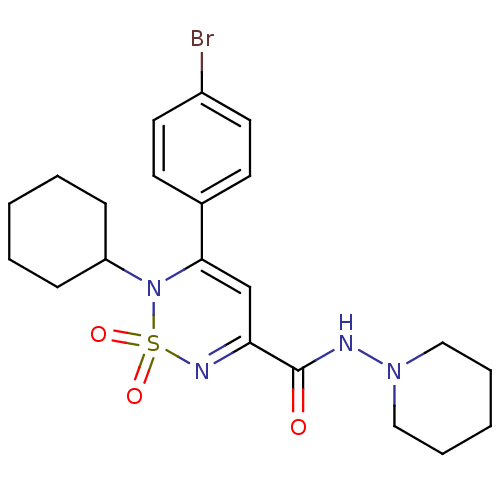 (CHEMBL391839 | N-(piperidin-1-yl)-3-(4-bromophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292324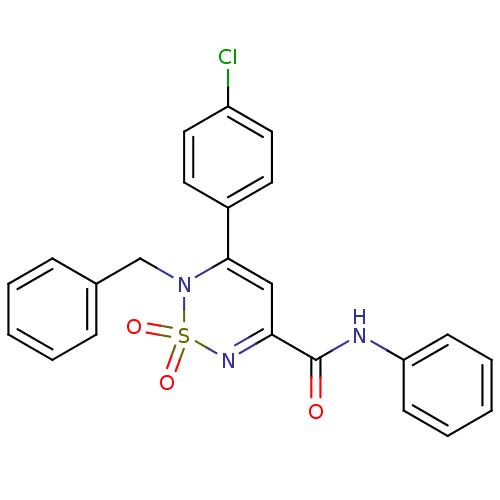 (CHEMBL393324 | N-phenyl-2-benzyl-3-(4-chlorophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.73E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||