 Found 185 hits with Last Name = 'gong' and Initial = 'hb'
Found 185 hits with Last Name = 'gong' and Initial = 'hb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25391
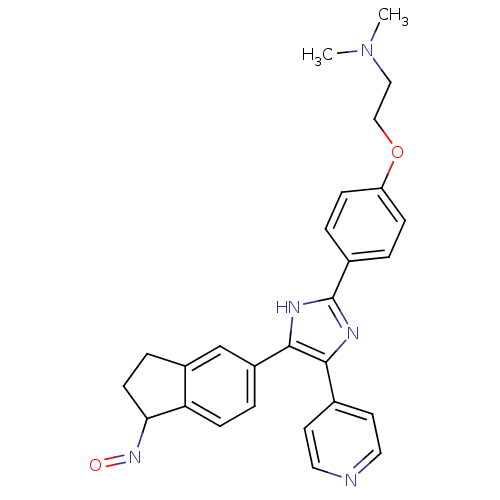
(CHEMBL200622 | SB-590885 | SB590885 | [2-(4-{4-[(1...)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc(c([nH]1)-c1ccc2C(CCc2c1)N=O)-c1ccncc1 Show InChI InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,24H,6,10,15-16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
Bioorg Med Chem 20: 3746-55 (2012)
Article DOI: 10.1016/j.bmc.2012.04.047
BindingDB Entry DOI: 10.7270/Q2QF8TXP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390673
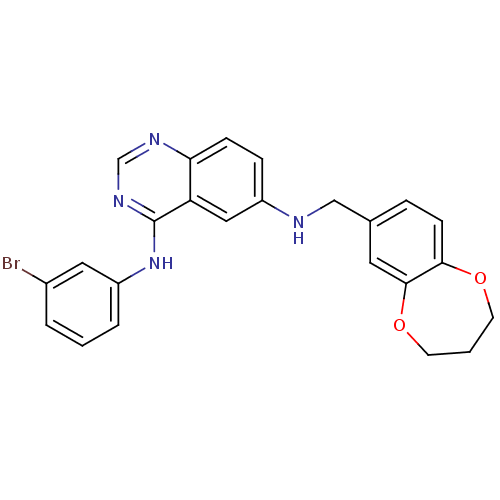
(CHEMBL2070198)Show SMILES Brc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCCOc5c4)cc23)c1 Show InChI InChI=1S/C24H21BrN4O2/c25-17-3-1-4-19(12-17)29-24-20-13-18(6-7-21(20)27-15-28-24)26-14-16-5-8-22-23(11-16)31-10-2-9-30-22/h1,3-8,11-13,15,26H,2,9-10,14H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 by chemiluminescent assay |
Bioorg Med Chem 20: 6648-54 (2012)
Article DOI: 10.1016/j.bmc.2012.09.021
BindingDB Entry DOI: 10.7270/Q2FX7BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383835
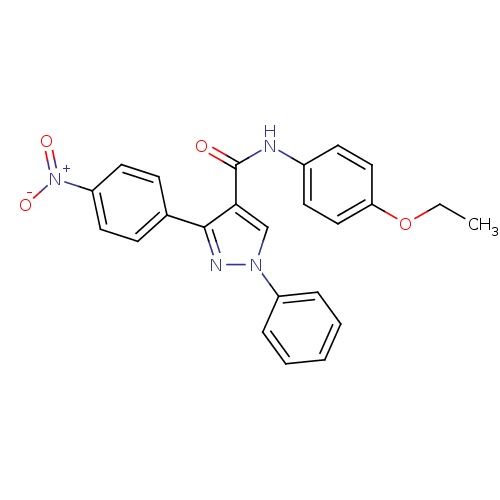
(CHEMBL2030993)Show SMILES CCOc1ccc(NC(=O)c2cn(nc2-c2ccc(cc2)[N+]([O-])=O)-c2ccccc2)cc1 Show InChI InChI=1S/C24H20N4O4/c1-2-32-21-14-10-18(11-15-21)25-24(29)22-16-27(19-6-4-3-5-7-19)26-23(22)17-8-12-20(13-9-17)28(30)31/h3-16H,2H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390674

(CHEMBL2070199)Show SMILES Clc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCCOc5c4)cc23)c1 Show InChI InChI=1S/C24H21ClN4O2/c25-17-3-1-4-19(12-17)29-24-20-13-18(6-7-21(20)27-15-28-24)26-14-16-5-8-22-23(11-16)31-10-2-9-30-22/h1,3-8,11-13,15,26H,2,9-10,14H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383830
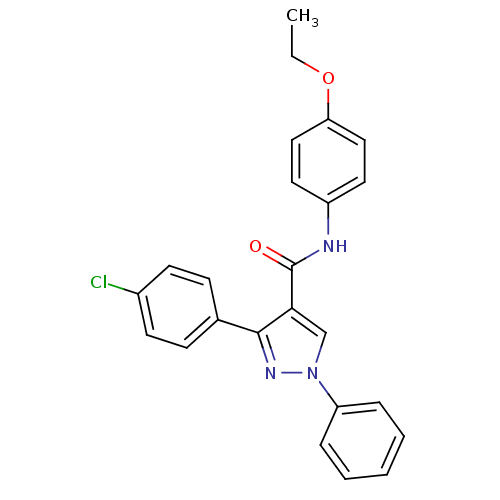
(CHEMBL2030988)Show SMILES CCOc1ccc(NC(=O)c2cn(nc2-c2ccc(Cl)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H20ClN3O2/c1-2-30-21-14-12-19(13-15-21)26-24(29)22-16-28(20-6-4-3-5-7-20)27-23(22)17-8-10-18(25)11-9-17/h3-16H,2H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM170126
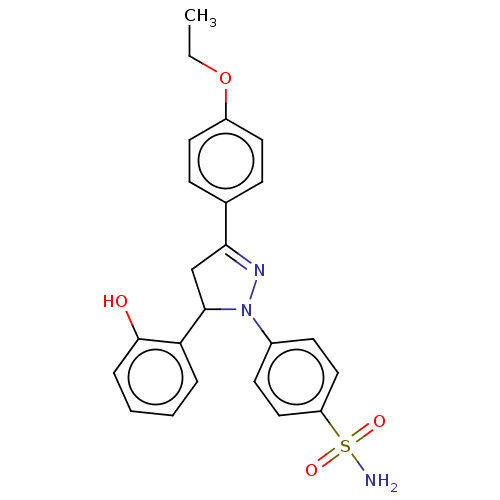
(4-(3-(4-Phenyl ethyl oxygen)-5-(2-hydroxyphenyl)-4...)Show SMILES CCOc1ccc(cc1)C1=NN(C(C1)c1ccccc1O)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C23H23N3O4S/c1-2-30-18-11-7-16(8-12-18)21-15-22(20-5-3-4-6-23(20)27)26(25-21)17-9-13-19(14-10-17)31(24,28)29/h3-14,22,27H,2,15H2,1H3,(H2,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
| Assay Description
This esterase assay used 4-nitrophenylacetate as a substrate. Initially, the rhMMP-2 was diluted to 100 µg/mL with 1 mM APMA in assay buffer. Later, ... |
Chem Biol Drug Des 86: 1405-10 (2015)
Article DOI: 10.1111/cbdd.12604
BindingDB Entry DOI: 10.7270/Q2XD10FB |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795
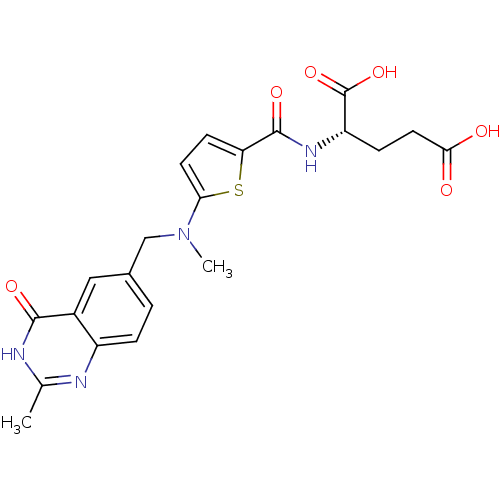
((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383816

(CHEMBL2030974)Show SMILES CCOc1ccc(NC(=O)c2cn(nc2-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H21N3O2/c1-2-29-21-15-13-19(14-16-21)25-24(28)22-17-27(20-11-7-4-8-12-20)26-23(22)18-9-5-3-6-10-18/h3-17H,2H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387112
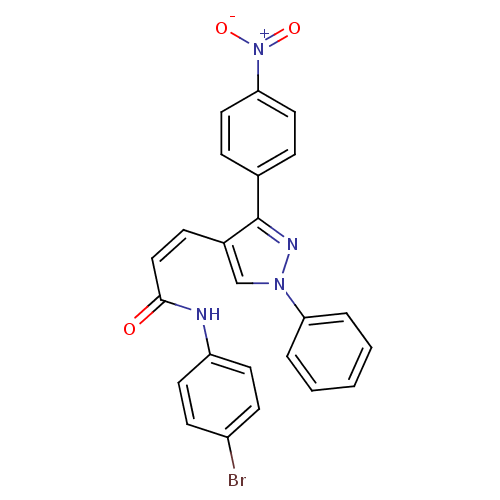
(CHEMBL2047619)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Br)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17BrN4O3/c25-19-9-11-20(12-10-19)26-23(30)15-8-18-16-28(21-4-2-1-3-5-21)27-24(18)17-6-13-22(14-7-17)29(31)32/h1-16H,(H,26,30)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387111
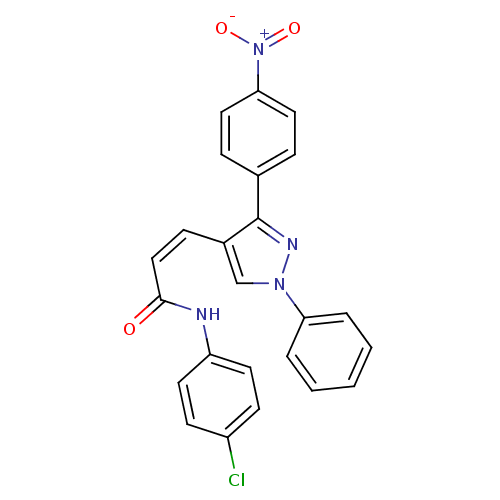
(CHEMBL2047618)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17ClN4O3/c25-19-9-11-20(12-10-19)26-23(30)15-8-18-16-28(21-4-2-1-3-5-21)27-24(18)17-6-13-22(14-7-17)29(31)32/h1-16H,(H,26,30)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50394508
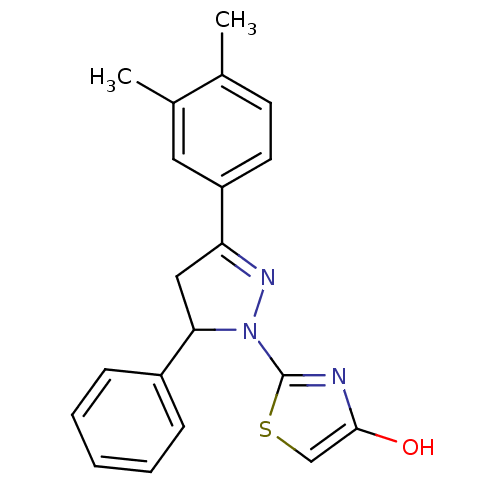
(CHEMBL2159921)Show SMILES Cc1ccc(cc1C)C1=NN(C(C1)c1ccccc1)c1nc(O)cs1 |t:9| Show InChI InChI=1S/C20H19N3OS/c1-13-8-9-16(10-14(13)2)17-11-18(15-6-4-3-5-7-15)23(22-17)20-21-19(24)12-25-20/h3-10,12,18,24H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 by chemiluminescent assay |
Bioorg Med Chem 20: 6648-54 (2012)
Article DOI: 10.1016/j.bmc.2012.09.021
BindingDB Entry DOI: 10.7270/Q2FX7BKC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of mouse wild type PI3Kalpha expressed in Sf21 cells co-expressing N-terminal His-tagged human p85a using L-alpha-phosphatidylinositol sub... |
Bioorg Med Chem 20: 3359-67 (2012)
Article DOI: 10.1016/j.bmc.2012.03.064
BindingDB Entry DOI: 10.7270/Q2MS3TR2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50387112

(CHEMBL2047619)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Br)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17BrN4O3/c25-19-9-11-20(12-10-19)26-23(30)15-8-18-16-28(21-4-2-1-3-5-21)27-24(18)17-6-13-22(14-7-17)29(31)32/h1-16H,(H,26,30)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390670

(CHEMBL2070191)Show SMILES Clc1cccc(Nc2ncnc3ccc(NCc4ccc5OCOc5c4)cc23)c1 Show InChI InChI=1S/C22H17ClN4O2/c23-15-2-1-3-17(9-15)27-22-18-10-16(5-6-19(18)25-12-26-22)24-11-14-4-7-20-21(8-14)29-13-28-20/h1-10,12,24H,11,13H2,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50387111

(CHEMBL2047618)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17ClN4O3/c25-19-9-11-20(12-10-19)26-23(30)15-8-18-16-28(21-4-2-1-3-5-21)27-24(18)17-6-13-22(14-7-17)29(31)32/h1-16H,(H,26,30)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta expressed in cells co-expressing N-terminal His-tagged human p85a using L-alpha-phosphatidylinositol substrate by TLC based p... |
Bioorg Med Chem 20: 3359-67 (2012)
Article DOI: 10.1016/j.bmc.2012.03.064
BindingDB Entry DOI: 10.7270/Q2MS3TR2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432391
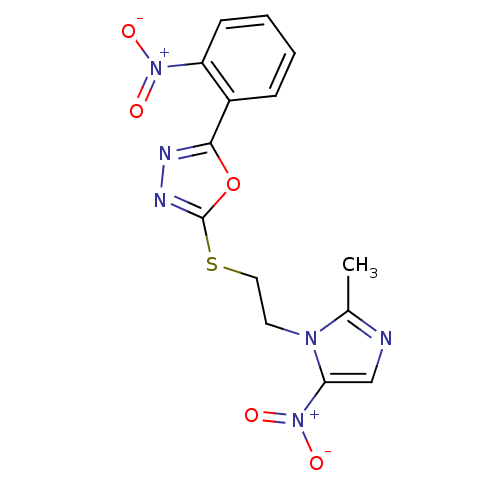
(CHEMBL2348578)Show SMILES Cc1ncc(n1CCSc1nnc(o1)-c1ccccc1[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C14H12N6O5S/c1-9-15-8-12(20(23)24)18(9)6-7-26-14-17-16-13(25-14)10-4-2-3-5-11(10)19(21)22/h2-5,8H,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390669

(CHEMBL2070197)Show SMILES Brc1cccc(Nc2ncnc3ccc(NCc4ccc5OCOc5c4)cc23)c1 Show InChI InChI=1S/C22H17BrN4O2/c23-15-2-1-3-17(9-15)27-22-18-10-16(5-6-19(18)25-12-26-22)24-11-14-4-7-20-21(8-14)29-13-28-20/h1-10,12,24H,11,13H2,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383836
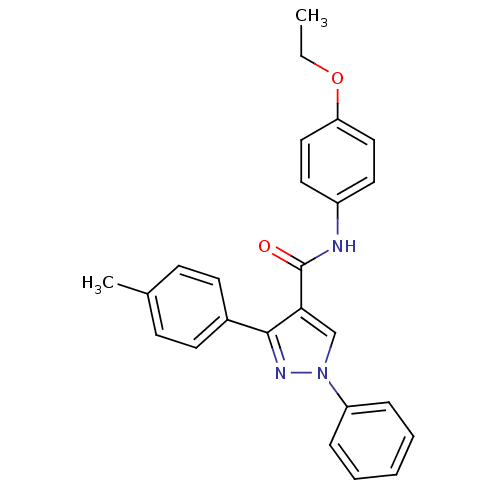
(CHEMBL2030978)Show SMILES CCOc1ccc(NC(=O)c2cn(nc2-c2ccc(C)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C25H23N3O2/c1-3-30-22-15-13-20(14-16-22)26-25(29)23-17-28(21-7-5-4-6-8-21)27-24(23)19-11-9-18(2)10-12-19/h4-17H,3H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383825

(CHEMBL2030983)Show SMILES CCOc1ccc(NC(=O)c2cn(nc2-c2ccc(OC)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C25H23N3O3/c1-3-31-22-15-11-19(12-16-22)26-25(29)23-17-28(20-7-5-4-6-8-20)27-24(23)18-9-13-21(30-2)14-10-18/h4-17H,3H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383834
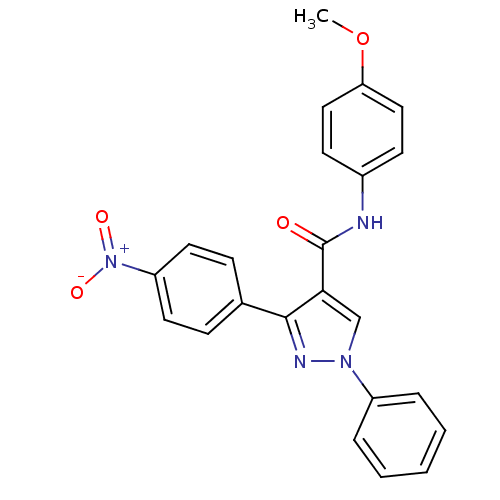
(CHEMBL2030992)Show SMILES COc1ccc(NC(=O)c2cn(nc2-c2ccc(cc2)[N+]([O-])=O)-c2ccccc2)cc1 Show InChI InChI=1S/C23H18N4O4/c1-31-20-13-9-17(10-14-20)24-23(28)21-15-26(18-5-3-2-4-6-18)25-22(21)16-7-11-19(12-8-16)27(29)30/h2-15H,1H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390672
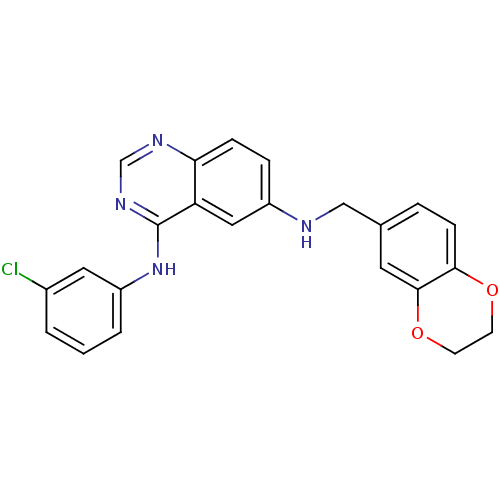
(CHEMBL2070193)Show SMILES Clc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCOc5c4)cc23)c1 Show InChI InChI=1S/C23H19ClN4O2/c24-16-2-1-3-18(11-16)28-23-19-12-17(5-6-20(19)26-14-27-23)25-13-15-4-7-21-22(10-15)30-9-8-29-21/h1-7,10-12,14,25H,8-9,13H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383829

(CHEMBL2030987)Show SMILES COc1ccc(NC(=O)c2cn(nc2-c2ccc(Cl)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H18ClN3O2/c1-29-20-13-11-18(12-14-20)25-23(28)21-15-27(19-5-3-2-4-6-19)26-22(21)16-7-9-17(24)10-8-16/h2-15H,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432387
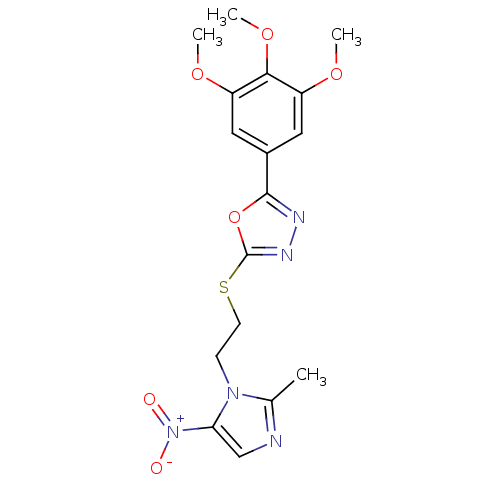
(CHEMBL2348559)Show SMILES COc1cc(cc(OC)c1OC)-c1nnc(SCCn2c(C)ncc2[N+]([O-])=O)o1 Show InChI InChI=1S/C17H19N5O6S/c1-10-18-9-14(22(23)24)21(10)5-6-29-17-20-19-16(28-17)11-7-12(25-2)15(27-4)13(8-11)26-3/h7-9H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432385
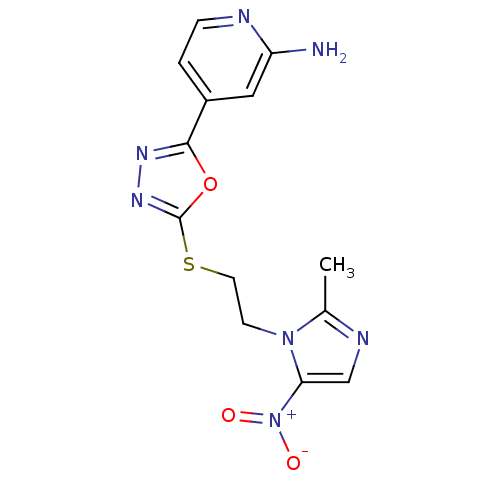
(CHEMBL2348566)Show SMILES Cc1ncc(n1CCSc1nnc(o1)-c1ccnc(N)c1)[N+]([O-])=O Show InChI InChI=1S/C13H13N7O3S/c1-8-16-7-11(20(21)22)19(8)4-5-24-13-18-17-12(23-13)9-2-3-15-10(14)6-9/h2-3,6-7H,4-5H2,1H3,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390676
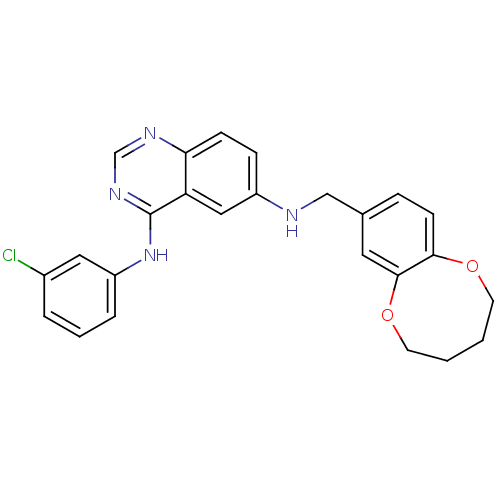
(CHEMBL2070201)Show SMILES Clc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCCCOc5c4)cc23)c1 Show InChI InChI=1S/C25H23ClN4O2/c26-18-4-3-5-20(13-18)30-25-21-14-19(7-8-22(21)28-16-29-25)27-15-17-6-9-23-24(12-17)32-11-2-1-10-31-23/h3-9,12-14,16,27H,1-2,10-11,15H2,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387110
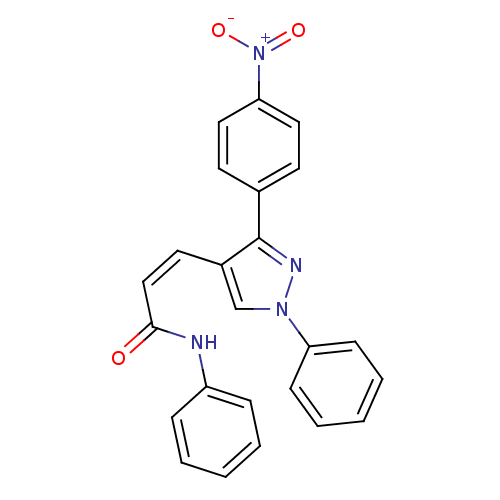
(CHEMBL2047617)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C24H18N4O3/c29-23(25-20-7-3-1-4-8-20)16-13-19-17-27(21-9-5-2-6-10-21)26-24(19)18-11-14-22(15-12-18)28(30)31/h1-17H,(H,25,29)/b16-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human wild type PI3Kbeta expressed in Sf21 cells co-expressing N-terminal His-tagged human p85a using L-alpha-phosphatidylinositol subs... |
Bioorg Med Chem 20: 3359-67 (2012)
Article DOI: 10.1016/j.bmc.2012.03.064
BindingDB Entry DOI: 10.7270/Q2MS3TR2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390671
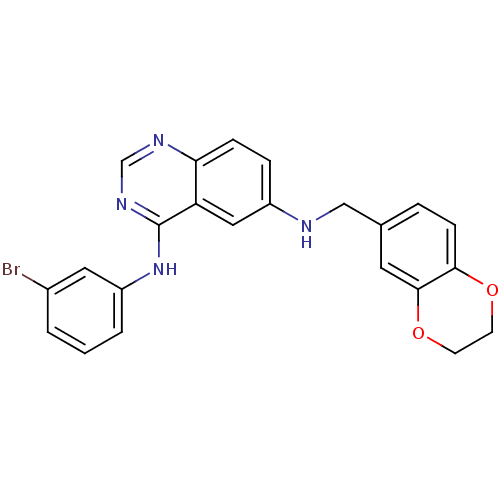
(CHEMBL2070192)Show SMILES Brc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCOc5c4)cc23)c1 Show InChI InChI=1S/C23H19BrN4O2/c24-16-2-1-3-18(11-16)28-23-19-12-17(5-6-20(19)26-14-27-23)25-13-15-4-7-21-22(10-15)30-9-8-29-21/h1-7,10-12,14,25H,8-9,13H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387113
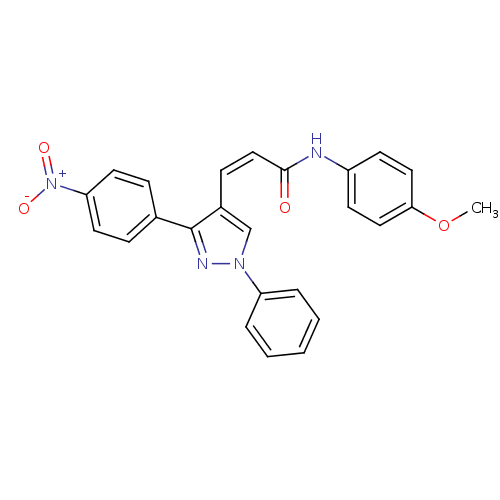
(CHEMBL2047620)Show SMILES COc1ccc(NC(=O)\C=C/c2cn(nc2-c2ccc(cc2)[N+]([O-])=O)-c2ccccc2)cc1 Show InChI InChI=1S/C25H20N4O4/c1-33-23-14-10-20(11-15-23)26-24(30)16-9-19-17-28(21-5-3-2-4-6-21)27-25(19)18-7-12-22(13-8-18)29(31)32/h2-17H,1H3,(H,26,30)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387105
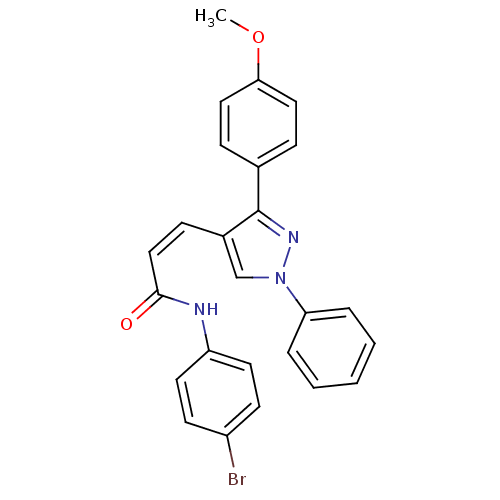
(CHEMBL2047611)Show SMILES COc1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Br)cc1)-c1ccccc1 Show InChI InChI=1S/C25H20BrN3O2/c1-31-23-14-7-18(8-15-23)25-19(17-29(28-25)22-5-3-2-4-6-22)9-16-24(30)27-21-12-10-20(26)11-13-21/h2-17H,1H3,(H,27,30)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387098
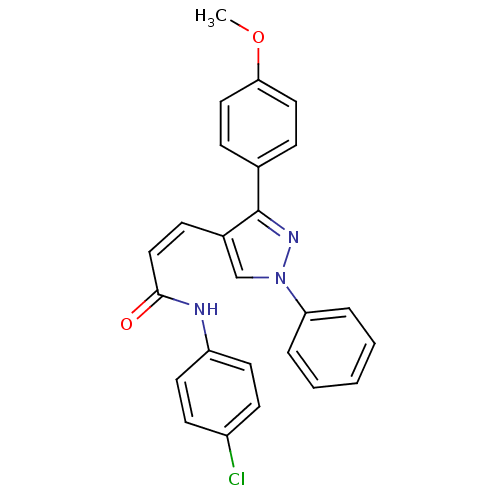
(CHEMBL2047610)Show SMILES COc1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C25H20ClN3O2/c1-31-23-14-7-18(8-15-23)25-19(17-29(28-25)22-5-3-2-4-6-22)9-16-24(30)27-21-12-10-20(26)11-13-21/h2-17H,1H3,(H,27,30)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM170149
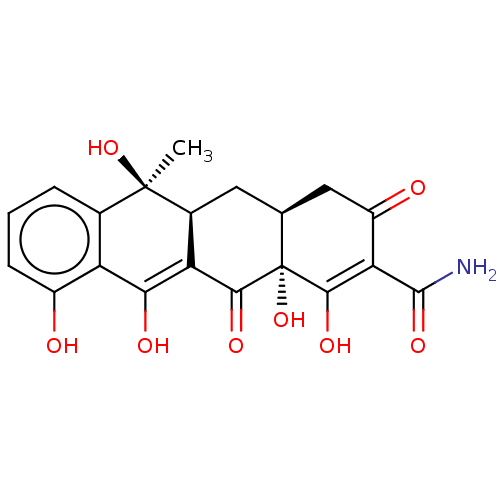
((4aS,5aS,6S,12aR)-1,6,10,11,12a-pentahydroxy-6-met...)Show SMILES C[C@]1(O)[C@H]2C[C@H]3CC(=O)C(C(N)=O)=C(O)[C@@]3(O)C(=O)C2=C(O)c2c(O)cccc12 |t:12,21| Show InChI InChI=1S/C20H19NO8/c1-19(28)8-3-2-4-10(22)12(8)15(24)13-9(19)5-7-6-11(23)14(18(21)27)17(26)20(7,29)16(13)25/h2-4,7,9,22,24,26,28-29H,5-6H2,1H3,(H2,21,27)/t7-,9-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
| Assay Description
This esterase assay used 4-nitrophenylacetate as a substrate. Initially, the rhMMP-2 was diluted to 100 µg/mL with 1 mM APMA in assay buffer. Later, ... |
Chem Biol Drug Des 86: 1405-10 (2015)
Article DOI: 10.1111/cbdd.12604
BindingDB Entry DOI: 10.7270/Q2XD10FB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390675

(CHEMBL2070200)Show SMILES Brc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCCCOc5c4)cc23)c1 Show InChI InChI=1S/C25H23BrN4O2/c26-18-4-3-5-20(13-18)30-25-21-14-19(7-8-22(21)28-16-29-25)27-15-17-6-9-23-24(12-17)32-11-2-1-10-31-23/h3-9,12-14,16,27H,1-2,10-11,15H2,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50387110

(CHEMBL2047617)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C24H18N4O3/c29-23(25-20-7-3-1-4-8-20)16-13-19-17-27(21-9-5-2-6-10-21)26-24(19)18-11-14-22(15-12-18)28(30)31/h1-17H,(H,25,29)/b16-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50387113

(CHEMBL2047620)Show SMILES COc1ccc(NC(=O)\C=C/c2cn(nc2-c2ccc(cc2)[N+]([O-])=O)-c2ccccc2)cc1 Show InChI InChI=1S/C25H20N4O4/c1-33-23-14-10-20(11-15-23)26-24(30)16-9-19-17-28(21-5-3-2-4-6-21)27-25(19)18-7-12-22(13-8-18)29(31)32/h2-17H,1H3,(H,26,30)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50394505
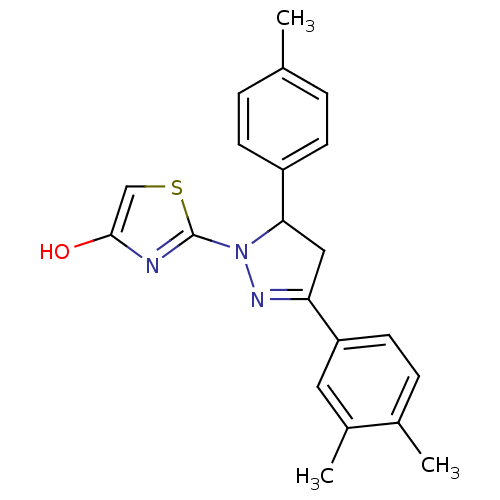
(CHEMBL2159925)Show SMILES Cc1ccc(cc1)C1CC(=NN1c1nc(O)cs1)c1ccc(C)c(C)c1 |c:10| Show InChI InChI=1S/C21H21N3OS/c1-13-4-7-16(8-5-13)19-11-18(17-9-6-14(2)15(3)10-17)23-24(19)21-22-20(25)12-26-21/h4-10,12,19,25H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 by chemiluminescent assay |
Bioorg Med Chem 20: 6648-54 (2012)
Article DOI: 10.1016/j.bmc.2012.09.021
BindingDB Entry DOI: 10.7270/Q2FX7BKC |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432386
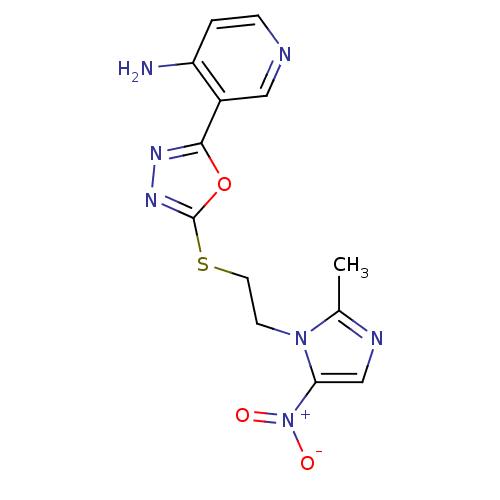
(CHEMBL2348563)Show SMILES Cc1ncc(n1CCSc1nnc(o1)-c1cnccc1N)[N+]([O-])=O Show InChI InChI=1S/C13H13N7O3S/c1-8-16-7-11(20(21)22)19(8)4-5-24-13-18-17-12(23-13)9-6-15-3-2-10(9)14/h2-3,6-7H,4-5H2,1H3,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390666
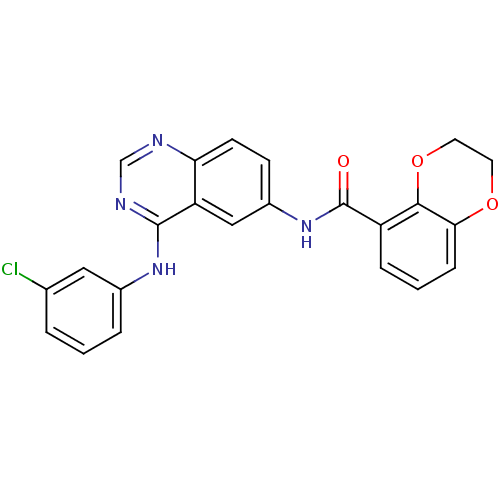
(CHEMBL2070194)Show SMILES Clc1cccc(Nc2ncnc3ccc(NC(=O)c4cccc5OCCOc45)cc23)c1 Show InChI InChI=1S/C23H17ClN4O3/c24-14-3-1-4-15(11-14)27-22-18-12-16(7-8-19(18)25-13-26-22)28-23(29)17-5-2-6-20-21(17)31-10-9-30-20/h1-8,11-13H,9-10H2,(H,28,29)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383815

(CHEMBL2030973)Show SMILES COc1ccc(NC(=O)c2cn(nc2-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H19N3O2/c1-28-20-14-12-18(13-15-20)24-23(27)21-16-26(19-10-6-3-7-11-19)25-22(21)17-8-4-2-5-9-17/h2-16H,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50388726
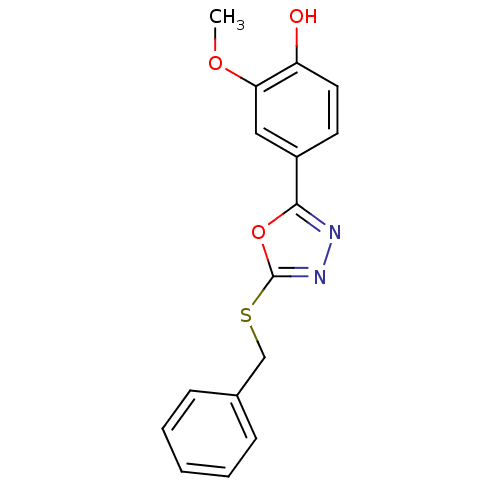
(CHEMBL2058568)Show InChI InChI=1S/C16H14N2O3S/c1-20-14-9-12(7-8-13(14)19)15-17-18-16(21-15)22-10-11-5-3-2-4-6-11/h2-9,19H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma after 4 hrs |
Bioorg Med Chem 20: 4226-36 (2012)
Article DOI: 10.1016/j.bmc.2012.05.055
BindingDB Entry DOI: 10.7270/Q2MK6DXD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383820
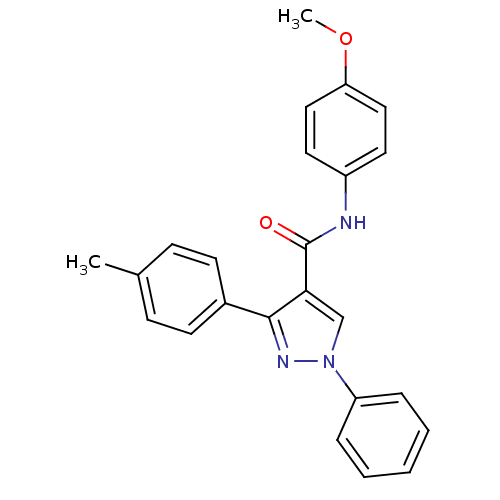
(CHEMBL1512579)Show SMILES COc1ccc(NC(=O)c2cn(nc2-c2ccc(C)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H21N3O2/c1-17-8-10-18(11-9-17)23-22(16-27(26-23)20-6-4-3-5-7-20)24(28)25-19-12-14-21(29-2)15-13-19/h3-16H,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432390
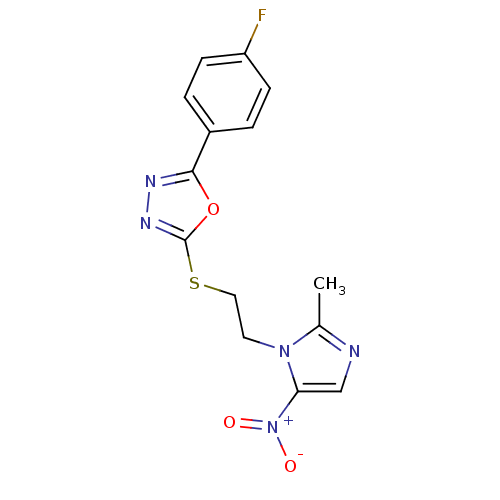
(CHEMBL2348548)Show SMILES Cc1ncc(n1CCSc1nnc(o1)-c1ccc(F)cc1)[N+]([O-])=O Show InChI InChI=1S/C14H12FN5O3S/c1-9-16-8-12(20(21)22)19(9)6-7-24-14-18-17-13(23-14)10-2-4-11(15)5-3-10/h2-5,8H,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50383824
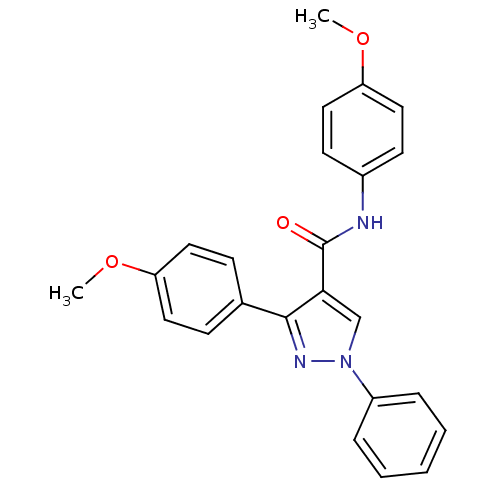
(CHEMBL2030982)Show SMILES COc1ccc(NC(=O)c2cn(nc2-c2ccc(OC)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H21N3O3/c1-29-20-12-8-17(9-13-20)23-22(16-27(26-23)19-6-4-3-5-7-19)24(28)25-18-10-14-21(30-2)15-11-18/h3-16H,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Aurora A kinase using biotinylated peptide substrate preincubated for 15 mins with compound measured after 30 mins by... |
Bioorg Med Chem Lett 22: 3589-93 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.066
BindingDB Entry DOI: 10.7270/Q2GF0VJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50390665

(CHEMBL2070190)Show SMILES Brc1cccc(Nc2ncnc3ccc(NC(=O)c4cccc5OCCOc45)cc23)c1 Show InChI InChI=1S/C23H17BrN4O3/c24-14-3-1-4-15(11-14)27-22-18-12-16(7-8-19(18)25-13-26-22)28-23(29)17-5-2-6-20-21(17)31-10-9-30-20/h1-8,11-13H,9-10H2,(H,28,29)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA |
Bioorg Med Chem Lett 22: 5870-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.079
BindingDB Entry DOI: 10.7270/Q26T0NRG |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50432383
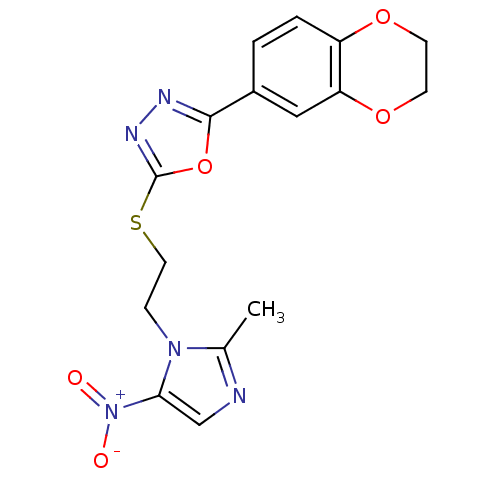
(CHEMBL2348547)Show SMILES Cc1ncc(n1CCSc1nnc(o1)-c1ccc2OCCOc2c1)[N+]([O-])=O Show InChI InChI=1S/C16H15N5O5S/c1-10-17-9-14(21(22)23)20(10)4-7-27-16-19-18-15(26-16)11-2-3-12-13(8-11)25-6-5-24-12/h2-3,8-9H,4-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase by spectrophotometric analysis |
Bioorg Med Chem 21: 2286-97 (2013)
Article DOI: 10.1016/j.bmc.2013.02.008
BindingDB Entry DOI: 10.7270/Q2FN17K3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387099
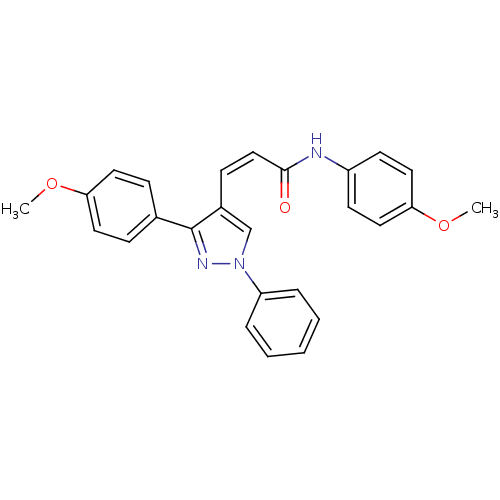
(CHEMBL2047612)Show SMILES COc1ccc(NC(=O)\C=C/c2cn(nc2-c2ccc(OC)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O3/c1-31-23-13-8-19(9-14-23)26-20(18-29(28-26)22-6-4-3-5-7-22)10-17-25(30)27-21-11-15-24(32-2)16-12-21/h3-18H,1-2H3,(H,27,30)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50387104
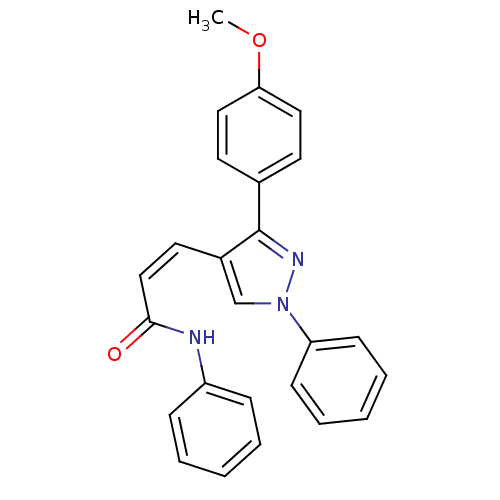
(CHEMBL2047609)Show SMILES COc1ccc(cc1)-c1nn(cc1\C=C/C(=O)Nc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C25H21N3O2/c1-30-23-15-12-19(13-16-23)25-20(18-28(27-25)22-10-6-3-7-11-22)14-17-24(29)26-21-8-4-2-5-9-21/h2-18H,1H3,(H,26,29)/b17-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cell lysate using KI-104 as substrate after 40 mins by fluorescence analysis |
Bioorg Med Chem 20: 4430-6 (2012)
Article DOI: 10.1016/j.bmc.2012.05.031
BindingDB Entry DOI: 10.7270/Q2416Z3D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data