

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50004352 (CHEMBL14925 | [(2R,5S)-5-(6-Amino-purin-9-yl)-tetr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier-2 des Sciences et Techniques du Languedoc Curated by ChEMBL | Assay Description inhibitory activity against Adenosine deaminase | J Med Chem 40: 3969-73 (1998) Article DOI: 10.1021/jm9701482 BindingDB Entry DOI: 10.7270/Q20002R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 2315-2320 (1995) Article DOI: 10.1016/0960-894X(95)00401-E BindingDB Entry DOI: 10.7270/Q2PG1S7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347260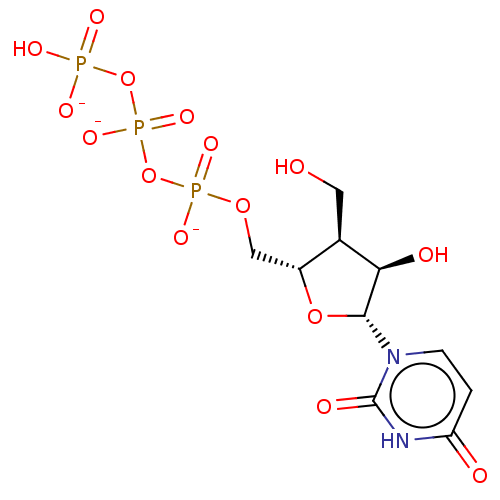 (US10202411, Compound 305) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347260 (US10202411, Compound 305) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347262 (US10202411, Compound 308) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347262 (US10202411, Compound 308) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347265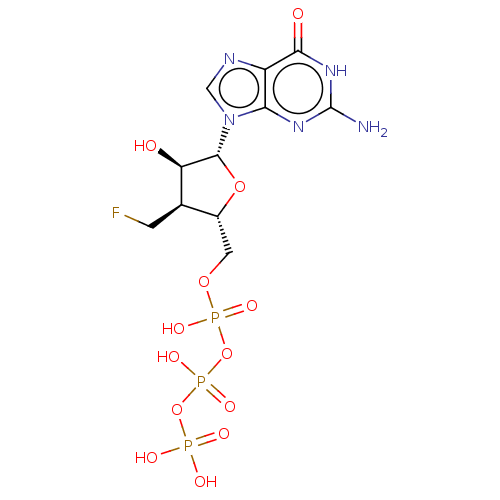 (US10202411, Compound 327) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347259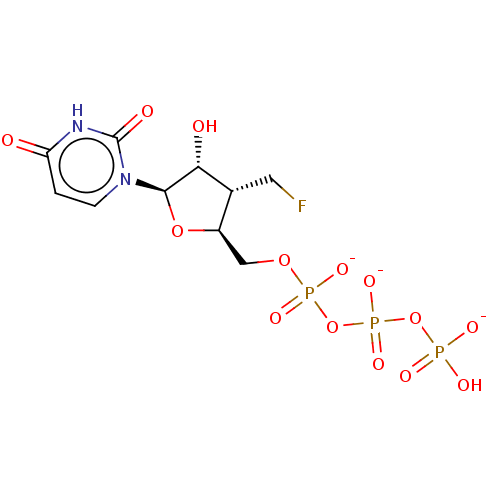 (US10202411, Compound 302) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347259 (US10202411, Compound 302) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347265 (US10202411, Compound 327) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319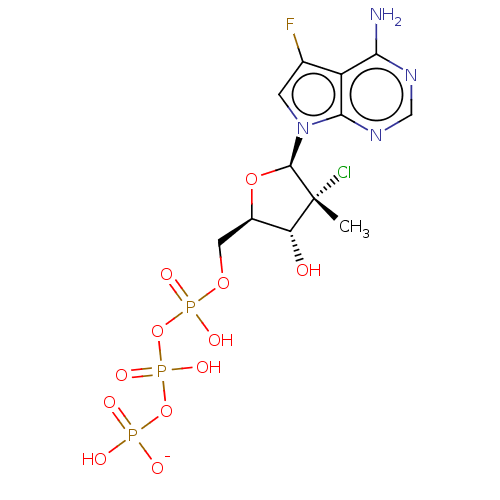 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318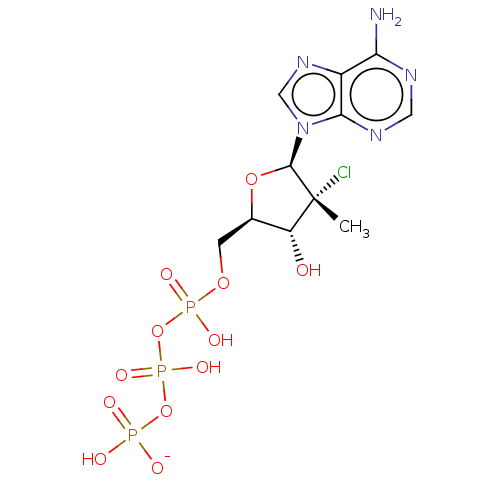 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347266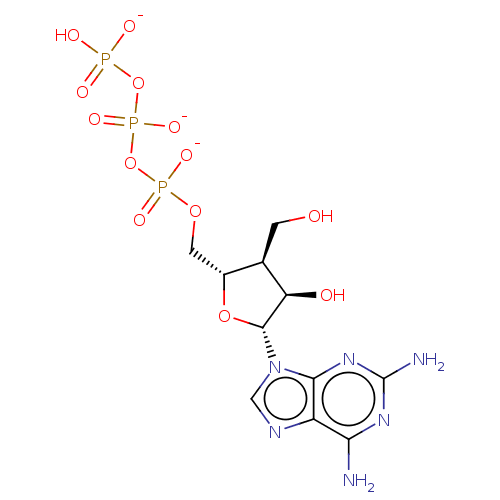 (US10202411, Compound 330) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312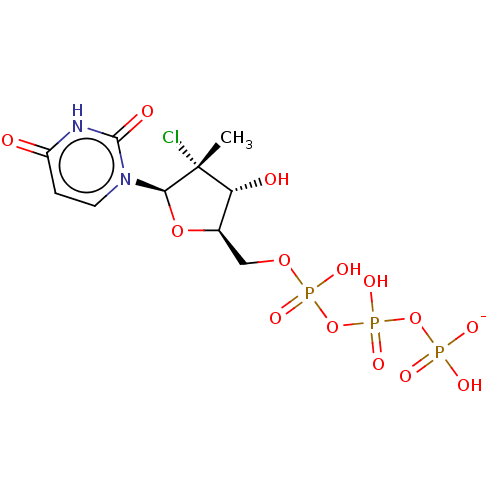 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317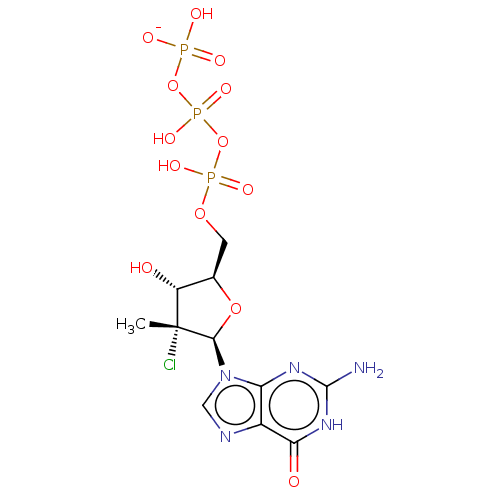 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347268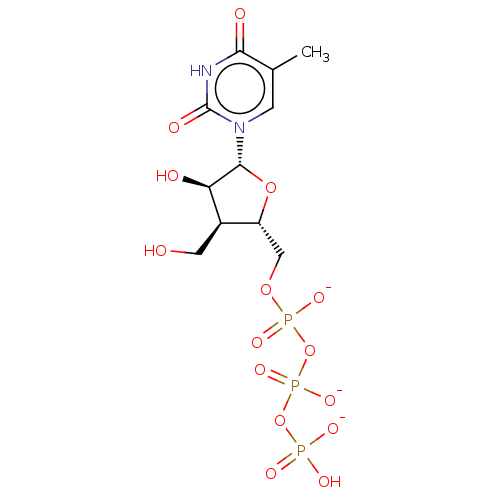 (US10202411, Compound 332) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347261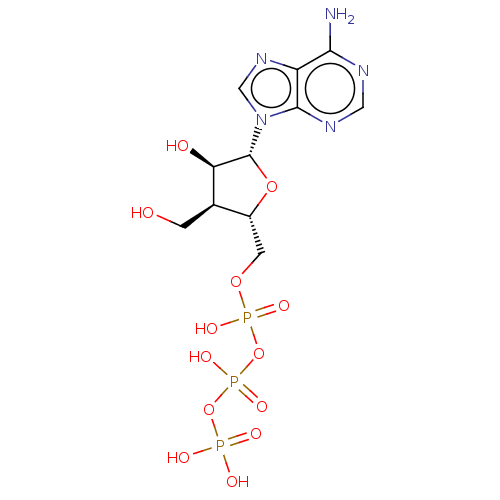 (US10202411, Compound 307) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347266 (US10202411, Compound 330) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347258 (US10202411, Compound 301) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347263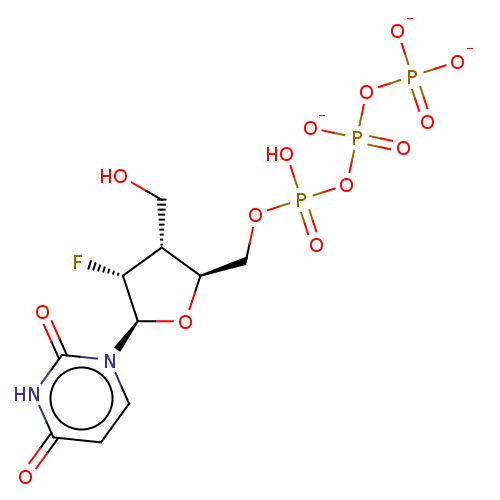 (US10202411, Compound 325) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347264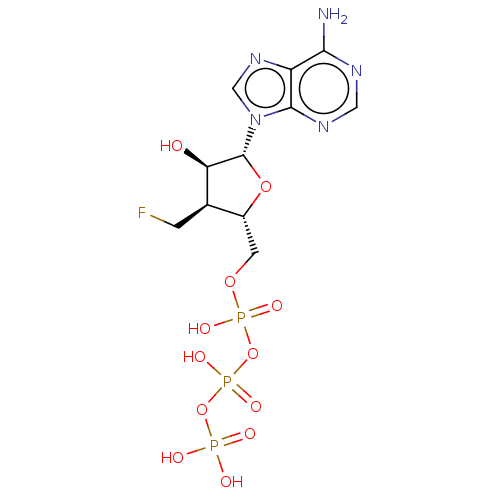 (US10202411, Compound 326) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347272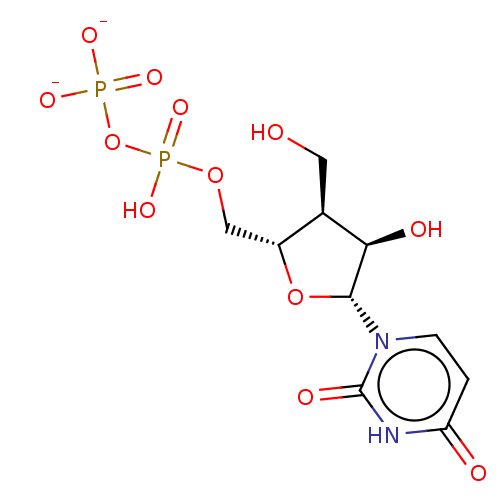 (US10202411, Compound 402) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426314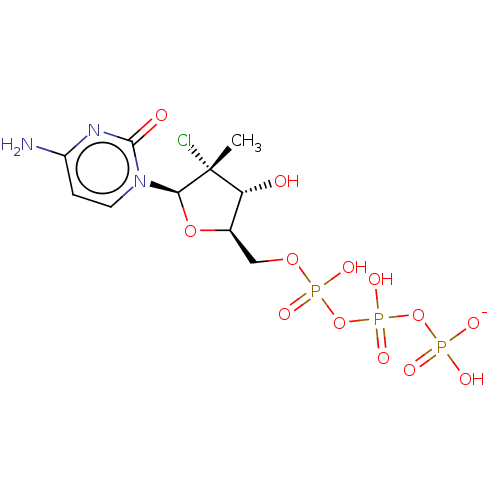 (US10513534, Compound 402) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426320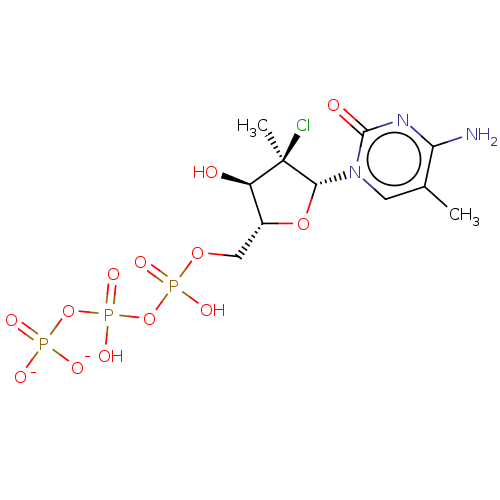 (US10513534, Compound 408) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426321 (US10513534, Compound 409) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426323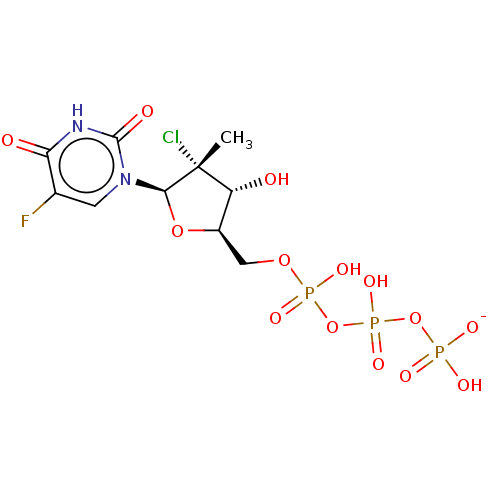 (US10513534, Compound 411) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426322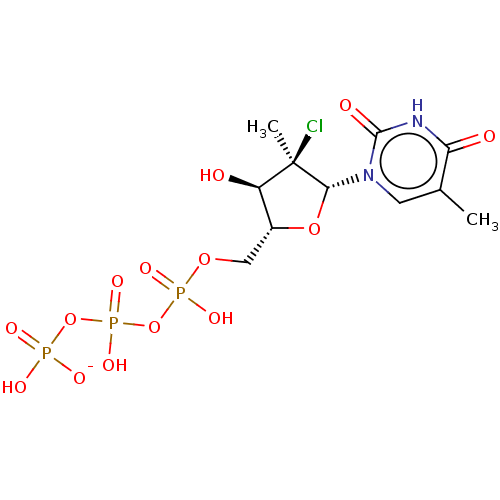 (US10513534, Compound 410) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347269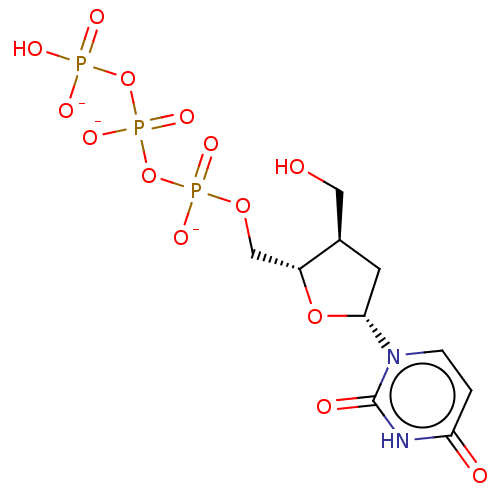 (US10202411, Compound 334) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347270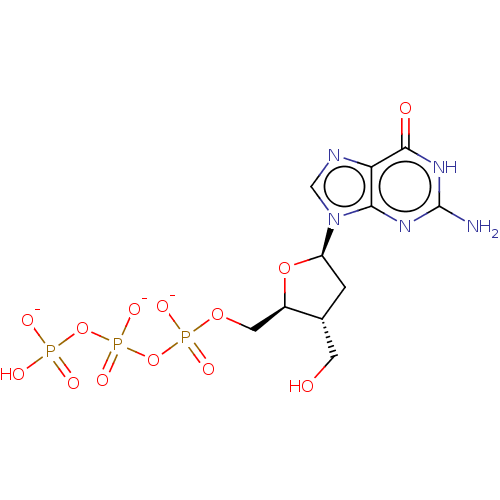 (US10202411, Compound 335) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347271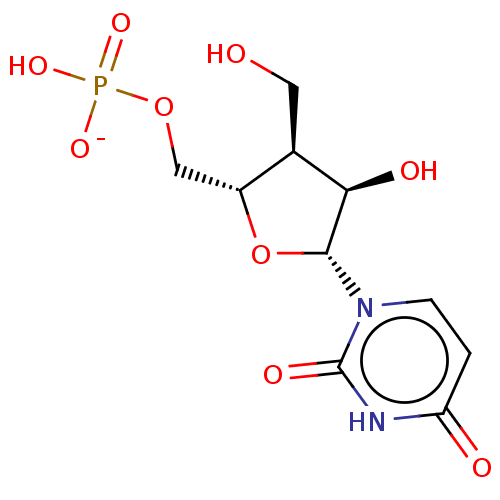 (US10202411, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426316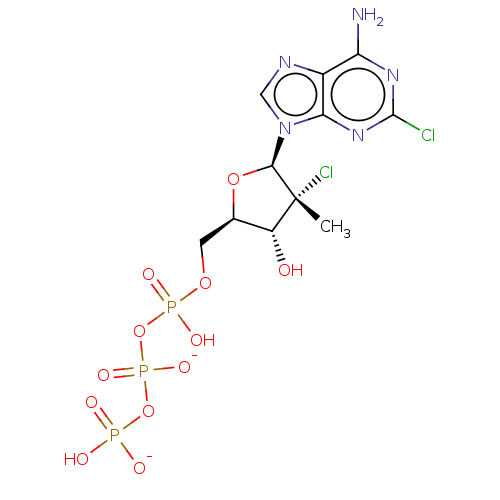 (US10513534, Compound 404) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347267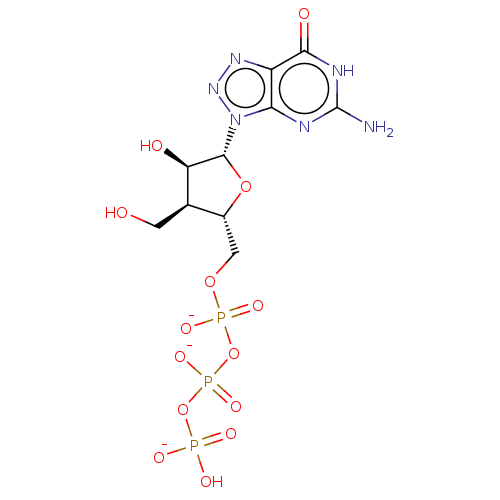 (US10202411, Compound 331) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426316 (US10513534, Compound 404) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50285469 (3-{[(2S,3R,5R)-3-Azido-5-(5-methyl-2,4-dioxo-3,4-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 2315-2320 (1995) Article DOI: 10.1016/0960-894X(95)00401-E BindingDB Entry DOI: 10.7270/Q2PG1S7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50285468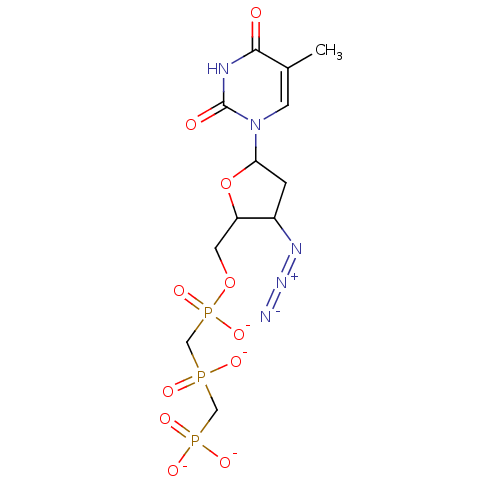 ({[(2S,3R,5R)-3-Azido-5-(5-methyl-2,4-dioxo-3,4-dih...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 5: 2315-2320 (1995) Article DOI: 10.1016/0960-894X(95)00401-E BindingDB Entry DOI: 10.7270/Q2PG1S7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50261285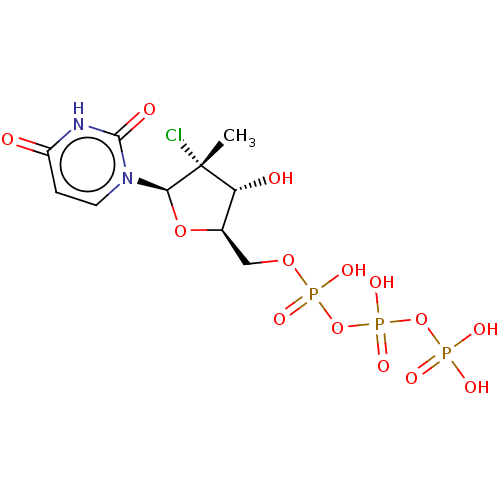 (CHEMBL4069349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX an MSD Company Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | Bioorg Med Chem Lett 27: 4323-4330 (2017) Article DOI: 10.1016/j.bmcl.2017.08.029 BindingDB Entry DOI: 10.7270/Q2MP55QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase, mitochondrial (Homo sapiens (Human)) | BDBM50261285 (CHEMBL4069349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX an MSD Company Curated by ChEMBL | Assay Description Inhibition of human mitochondrial RNA polymerase | Bioorg Med Chem Lett 27: 4323-4330 (2017) Article DOI: 10.1016/j.bmcl.2017.08.029 BindingDB Entry DOI: 10.7270/Q2MP55QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50261285 (CHEMBL4069349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX an MSD Company Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha | Bioorg Med Chem Lett 27: 4323-4330 (2017) Article DOI: 10.1016/j.bmcl.2017.08.029 BindingDB Entry DOI: 10.7270/Q2MP55QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194159 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194162 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194163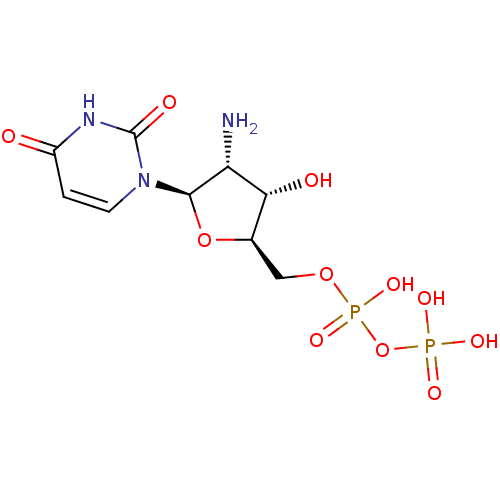 ((2R,3R,4S,5R)-1-(3-amino-5-(diphosphoryloxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194164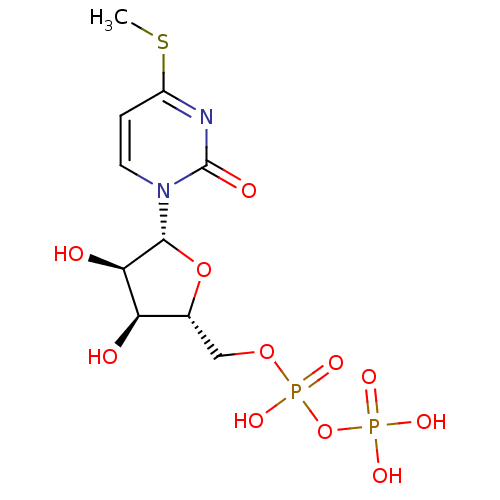 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50194161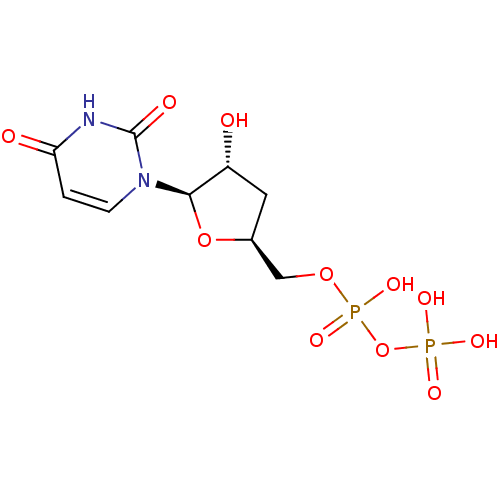 ((2R,3R,5S)-1-(5-(diphosphoryloxymethyl)-3-hydroxyt...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194167 ((2R,3R,4S,5R)-4-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |