

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264124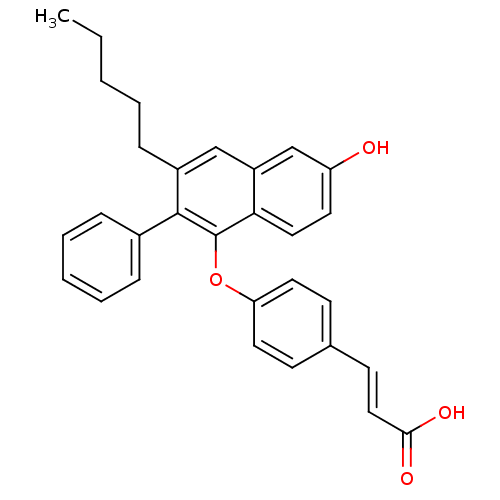 (3-(4-(6-hydroxy-3-pentyl-2-phenylnaphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264124 (3-(4-(6-hydroxy-3-pentyl-2-phenylnaphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264122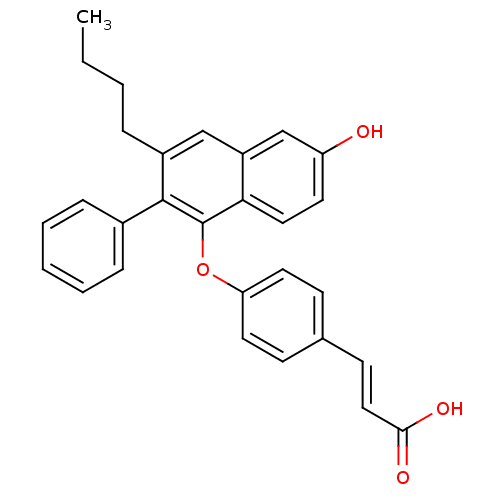 (3-(4-(3-butyl-6-hydroxy-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264122 (3-(4-(3-butyl-6-hydroxy-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264121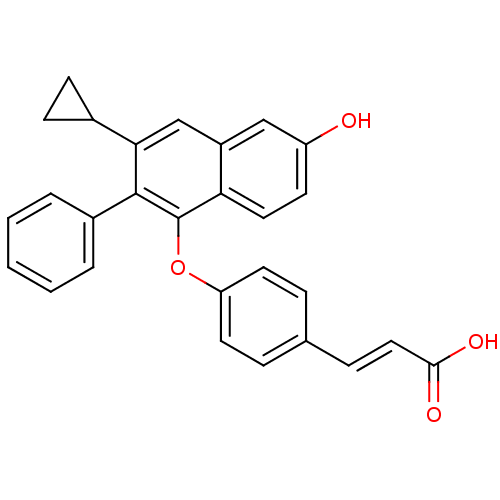 (3-(4-(3-cyclopropyl-6-hydroxy-2-phenylnaphthalen-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474916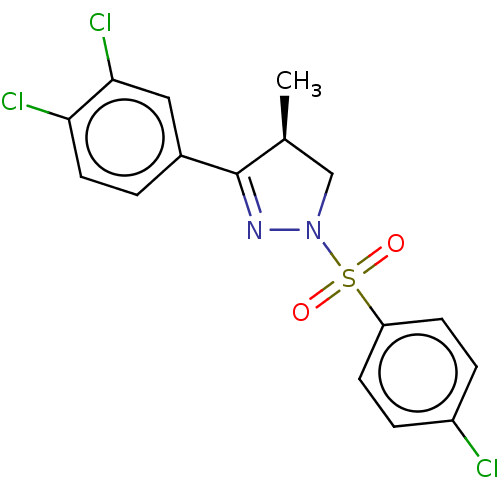 (CHEMBL364712) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264123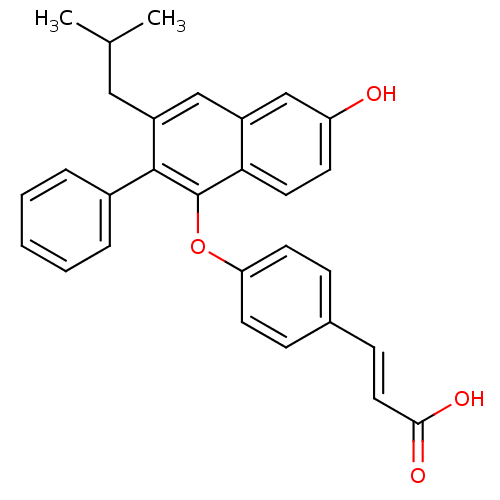 (3-(4-(6-hydroxy-3-isobutyl-2-phenylnaphthalen-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474916 (CHEMBL364712) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264123 (3-(4-(6-hydroxy-3-isobutyl-2-phenylnaphthalen-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264172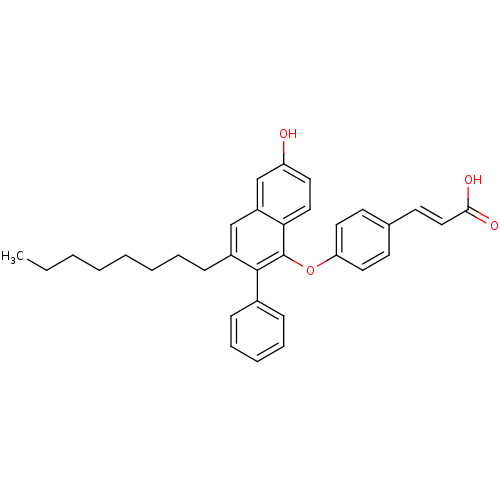 (3-(4-(6-hydroxy-3-octyl-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474911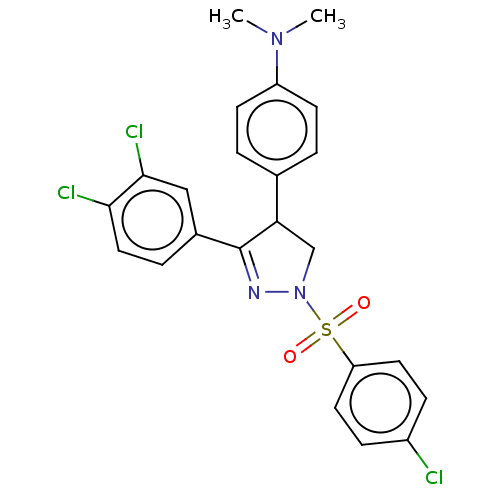 (CHEMBL192314) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474914 (CHEMBL190198) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474917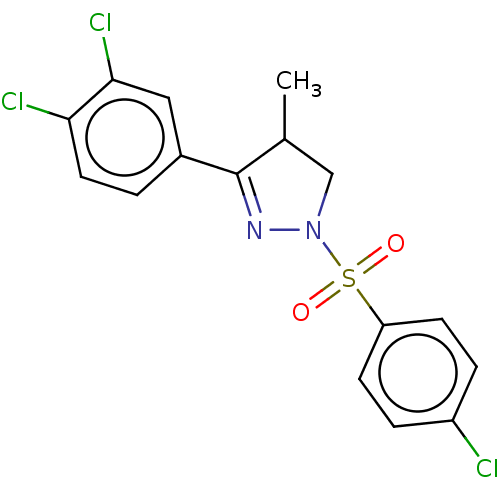 (CHEMBL366252) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264695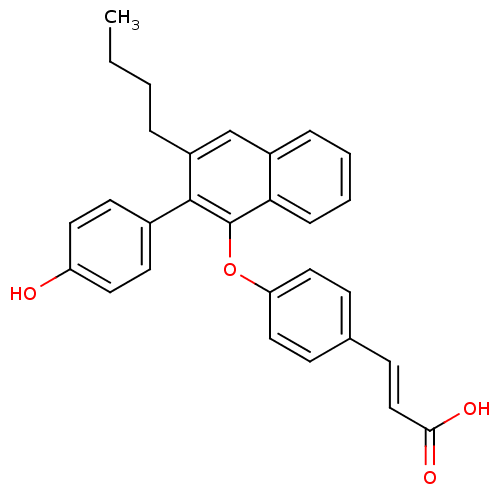 (3-(4-(3-butyl-2-(4-hydroxyphenyl)naphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264695 (3-(4-(3-butyl-2-(4-hydroxyphenyl)naphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264172 (3-(4-(6-hydroxy-3-octyl-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264121 (3-(4-(3-cyclopropyl-6-hydroxy-2-phenylnaphthalen-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474912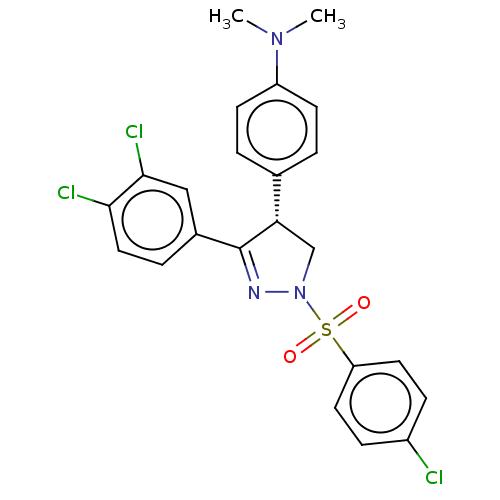 (CHEMBL190264) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474919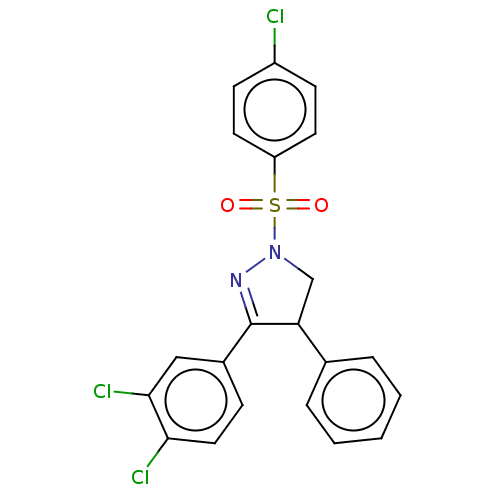 (CHEMBL364924) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264693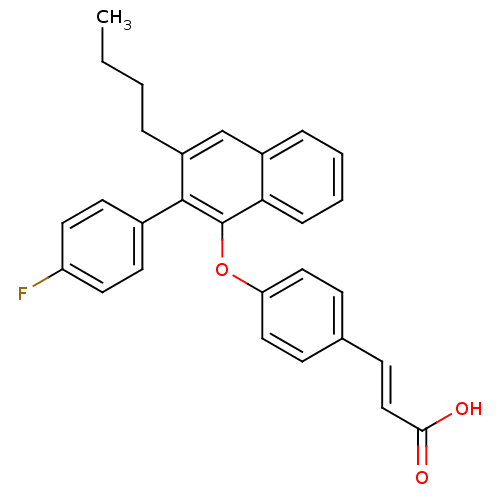 (3-(4-(3-butyl-2-(4-fluorophenyl)naphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264694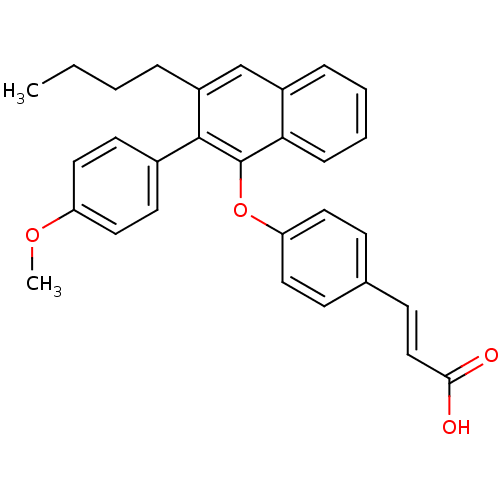 (3-(4-(3-butyl-2-(4-methoxyphenyl)naphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264694 (3-(4-(3-butyl-2-(4-methoxyphenyl)naphthalen-1-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264692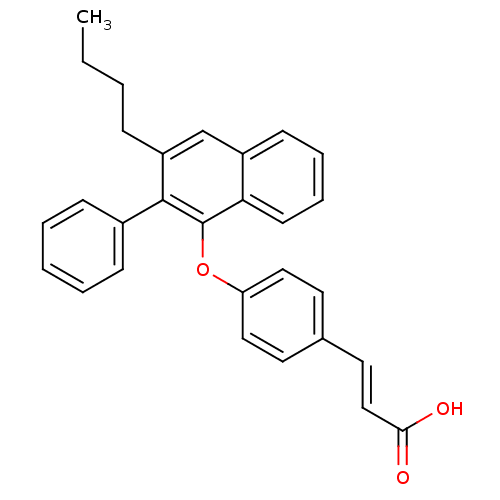 (3-(4-(3-butyl-2-phenylnaphthalen-1-yloxy)phenyl)ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474914 (CHEMBL190198) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474915 (CHEMBL190900) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50440737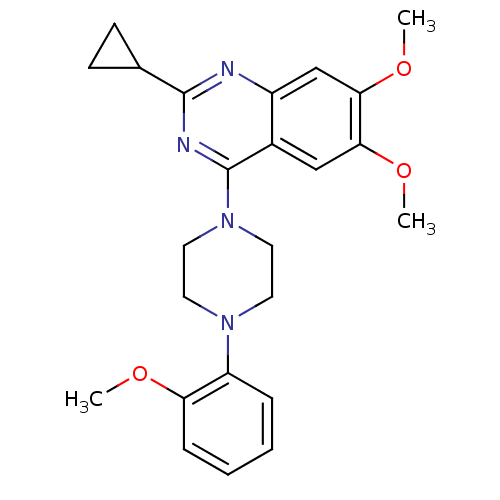 (CHEMBL2431120) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50264660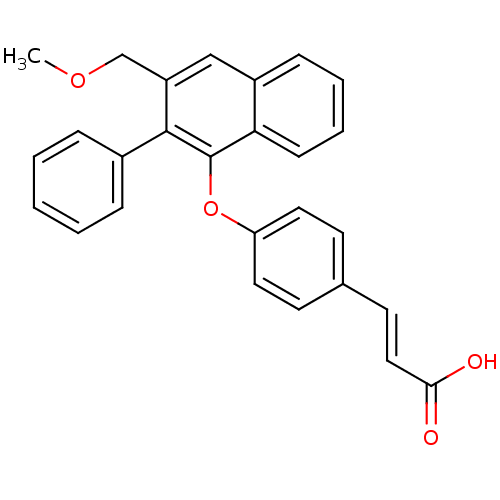 (3-(4-(3-(methoxymethyl)-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264660 (3-(4-(3-(methoxymethyl)-2-phenylnaphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264693 (3-(4-(3-butyl-2-(4-fluorophenyl)naphthalen-1-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474911 (CHEMBL192314) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50264692 (3-(4-(3-butyl-2-phenylnaphthalen-1-yloxy)phenyl)ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 18: 5075-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.121 BindingDB Entry DOI: 10.7270/Q2C53KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474912 (CHEMBL190264) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474913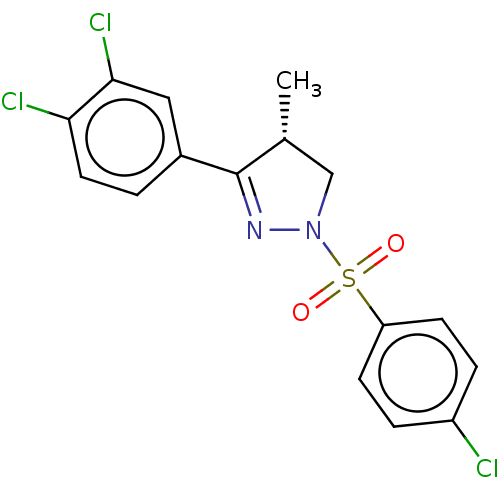 (CHEMBL192859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to PBR receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474918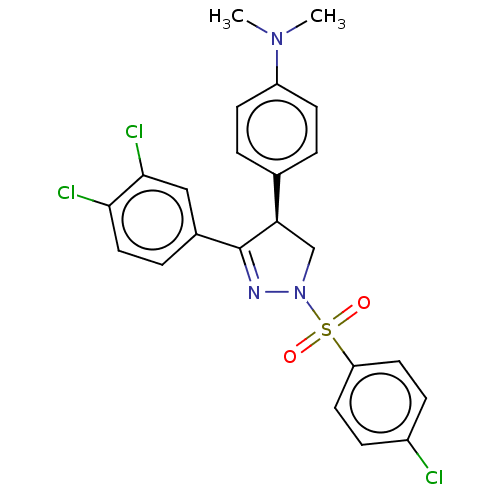 (CHEMBL373299) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of MOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474913 (CHEMBL192859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50474915 (CHEMBL190900) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of fluorescent ligand from binding domain of progesterone receptor | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943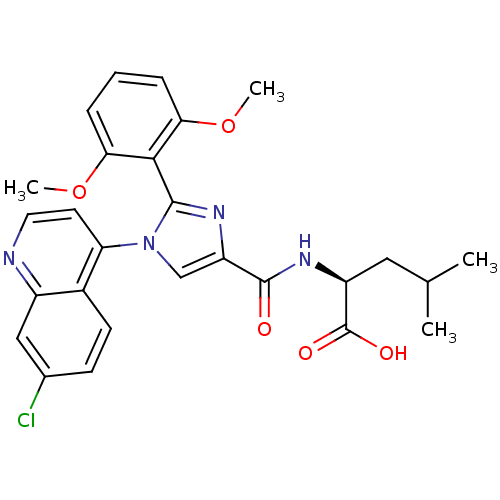 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 346 total ) | Next | Last >> |