

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50347819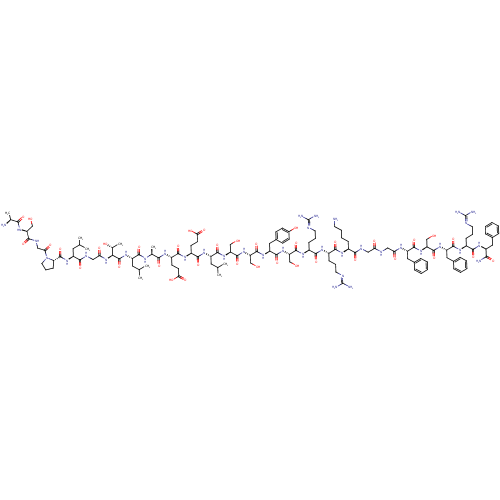 (CHEMBL1802414 | P550) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50347818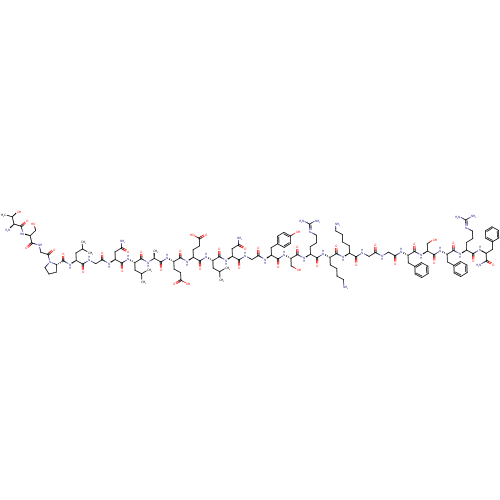 (CHEMBL1802413 | P518) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86228 (P517) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86226 (P52) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86224 (P513) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86227 (P552) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 607 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86225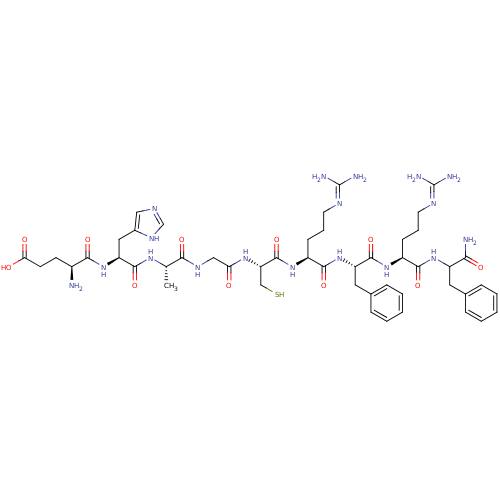 (P51) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM86229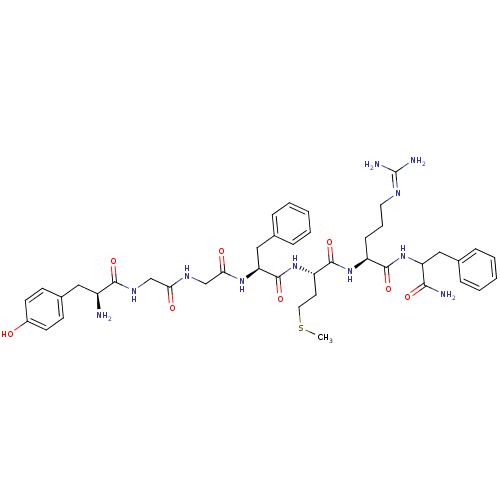 (YGGFMRF-amide) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by PDSP Ki Database | J Biol Chem 278: 27652-7 (2003) Article DOI: 10.1074/jbc.M302945200 BindingDB Entry DOI: 10.7270/Q2ZK5F81 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129287 (US8815951, 104) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129278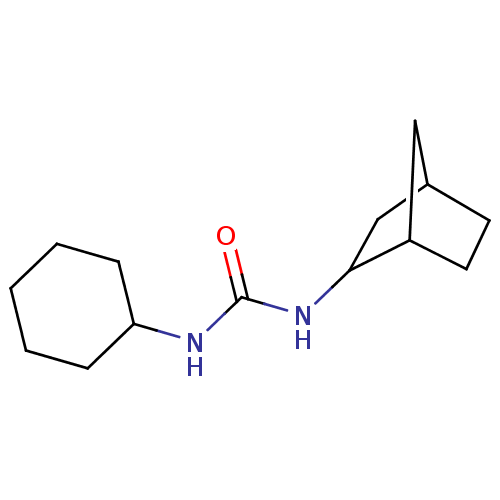 (US8815951, 543) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM25732 (3-adamantan-1-yl-1-cyclohexylurea | CHEMBL242255 |...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129279 (US8815951, 427) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129280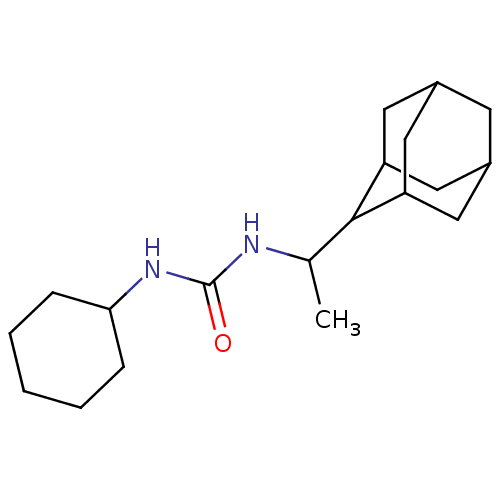 (US8815951, 358) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129281 (US8815951, 435) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129282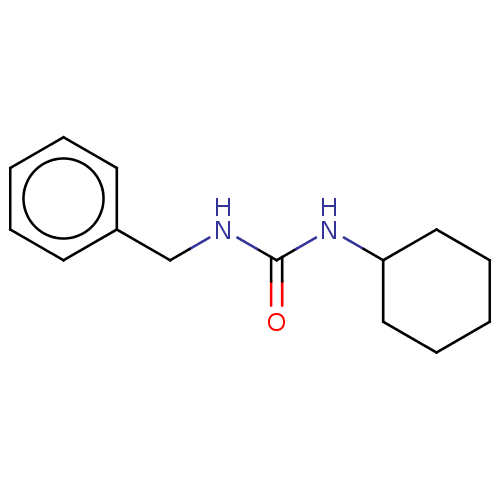 (US8815951, 270) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129283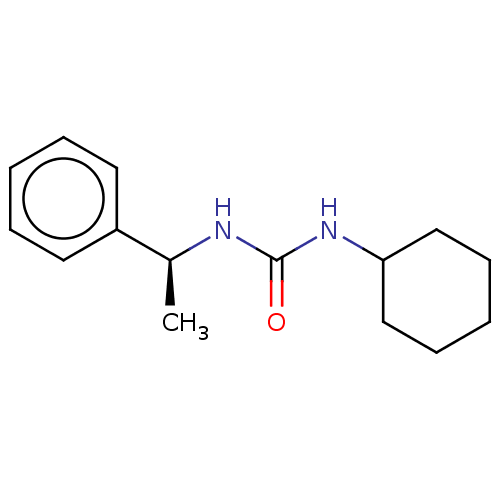 (US8815951, 544) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129284 (US8815951, 545) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129285 (US8815951, 437) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129288 (US8815951, 105) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129291 (US8815951, 434) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129295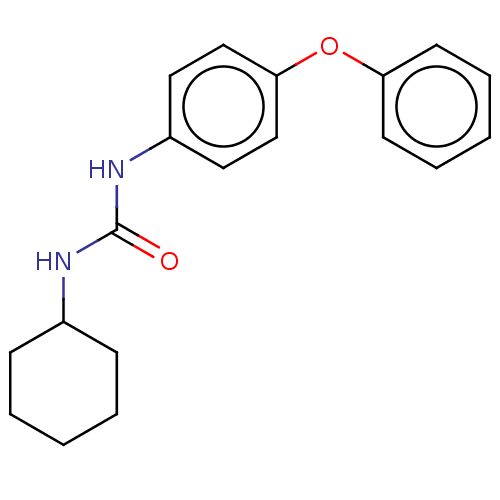 (US8815951, 140) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129297 (US8815951, 384) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM50383484 (CHEMBL2031931 | US8815951, 343) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM50383505 (CHEMBL2031932 | US8815951, 501) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129301 (US8815951, 193) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129306 (US8815951, 379) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129307 (US8815951, 362) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129310 (US8815951, 411) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129311 (US8815951, 412) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129312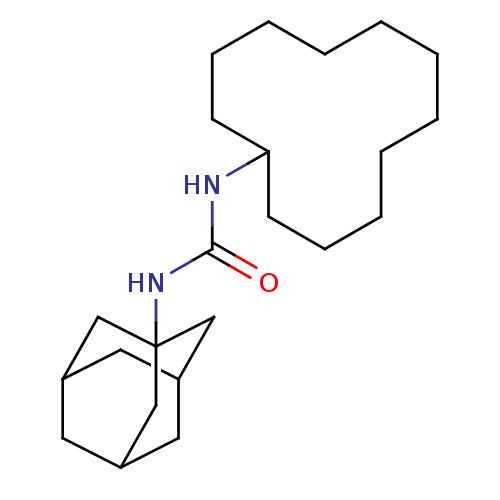 (US8815951, 413) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM50383476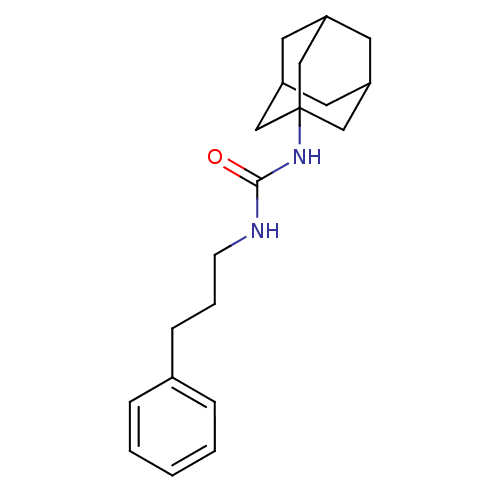 (CHEMBL2031923 | US8815951, 438) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129322 (US8815951, 257) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129223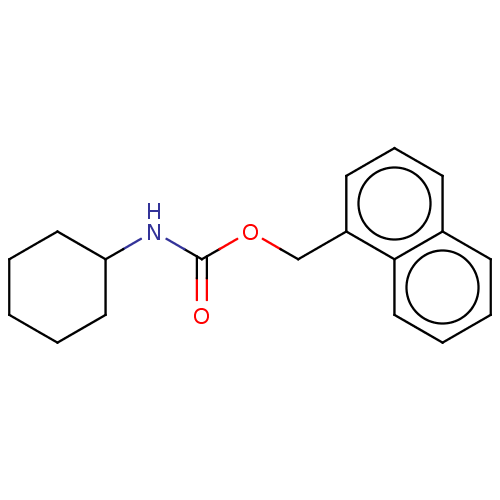 (US8815951, 262) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129228 (US8815951, 187) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129249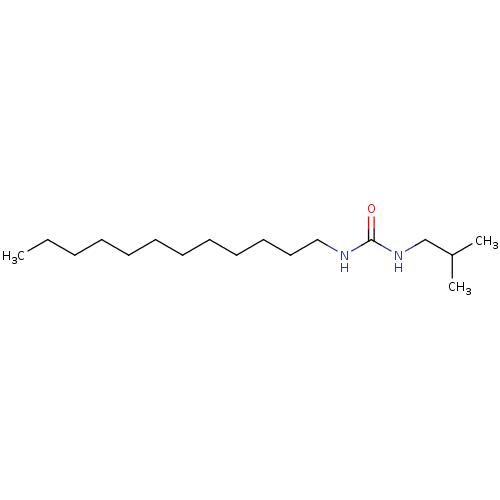 (US8815951, 478) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129255 (US8815951, 124) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129258 (US8815951, 344) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129259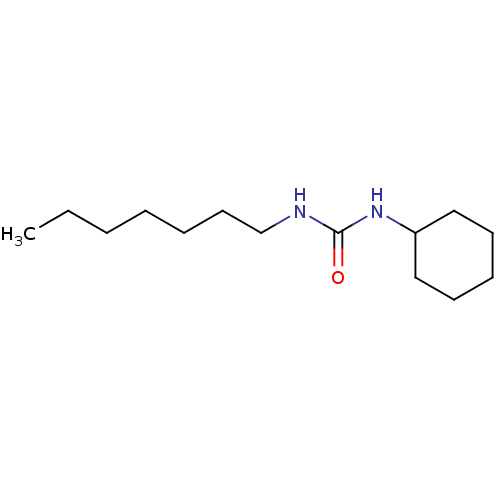 (US8815951, 508) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129260 (US8815951, 473) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129261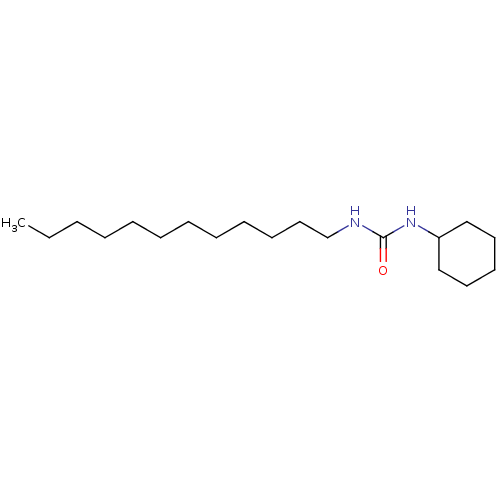 (US8815951, 297) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129262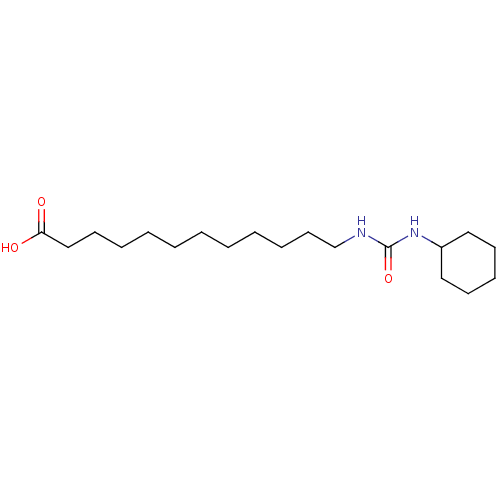 (US8815951, 425) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129263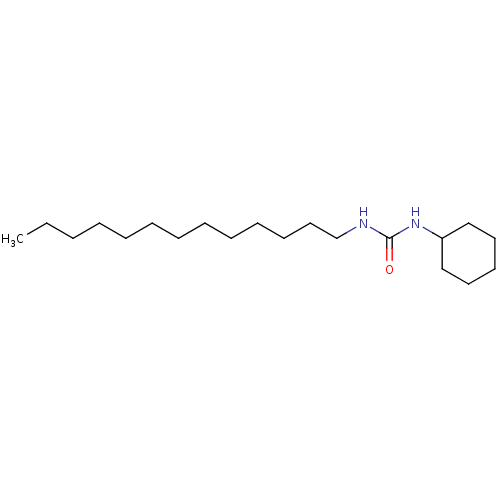 (US8815951, 354) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129266 (US8815951, 538) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129267 (US8815951, 551) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129269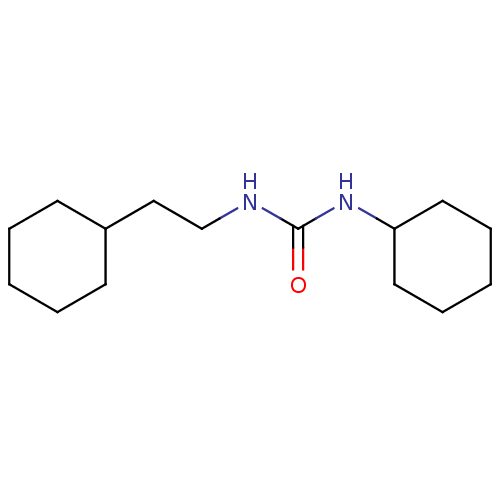 (US8815951, 360) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129270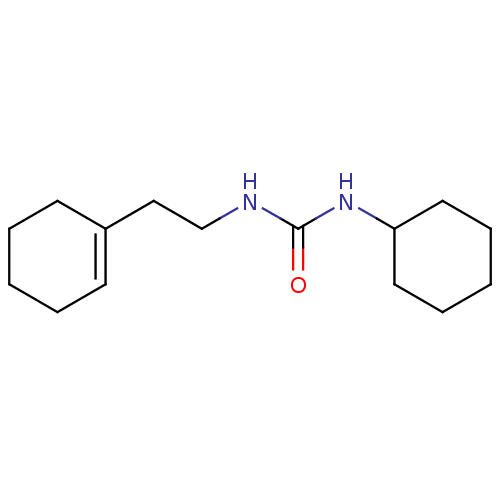 (US8815951, 359) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129272 (US8815951, 533) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129275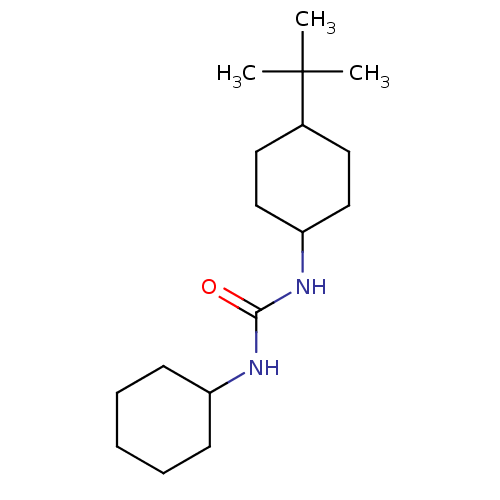 (US8815951, 428) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM129276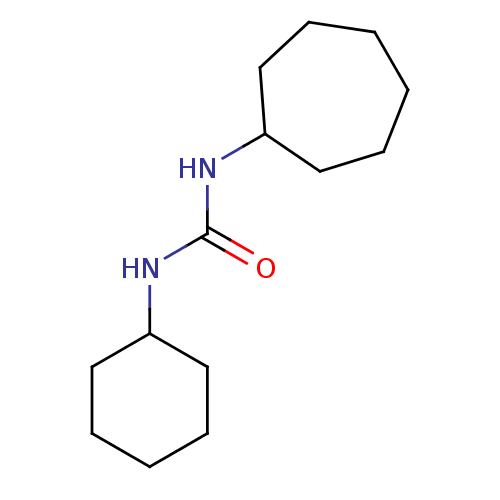 (US8815951, 22) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 1 (Mus musculus (Mouse)) | BDBM41921 (1-cyclohexyl-3-cyclooctyl-urea | 1-cyclohexyl-3-cy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description The invention also provide methods for assaying for epoxide hydrolase activity as diagnostic assay to identify individuals at increased risk for hype... | US Patent US8815951 (2014) BindingDB Entry DOI: 10.7270/Q2BP01G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 373 total ) | Next | Last >> |