

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.205 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130909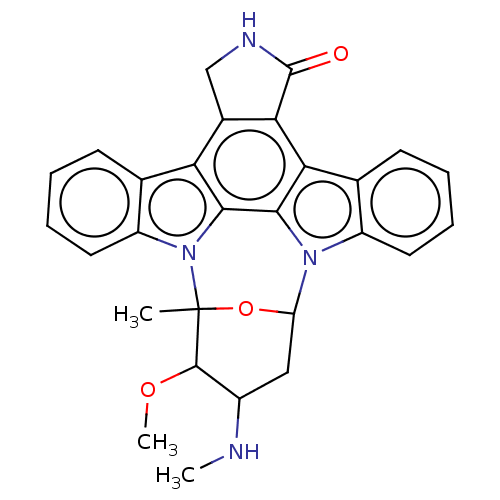 (US10683289, Example Staurosporine | US10927120, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130911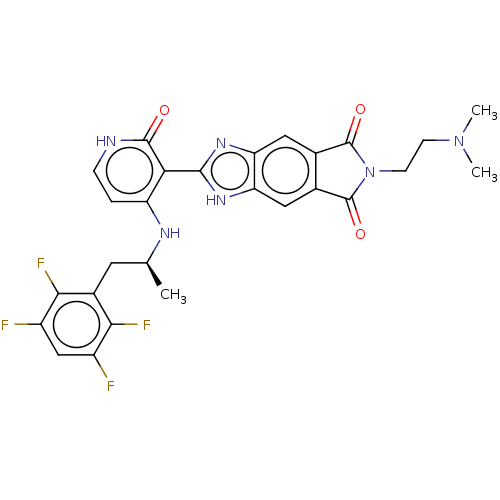 (US8822500, [2]) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.436 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130919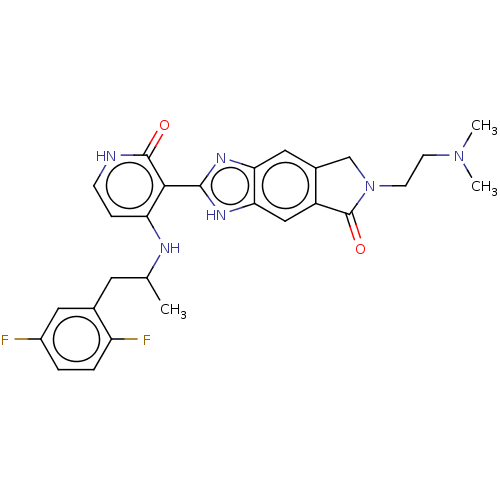 (US8822500, [18]) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.688 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8986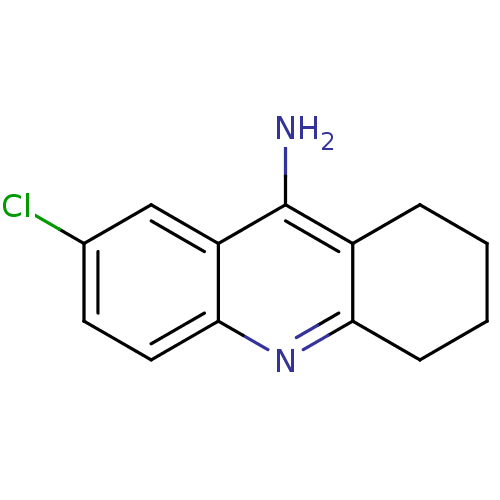 (7-chloro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50060470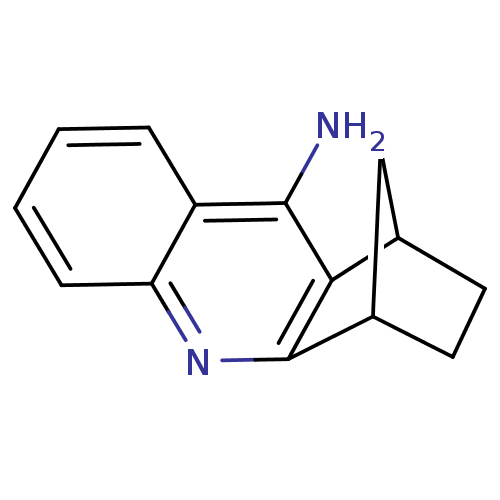 (10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987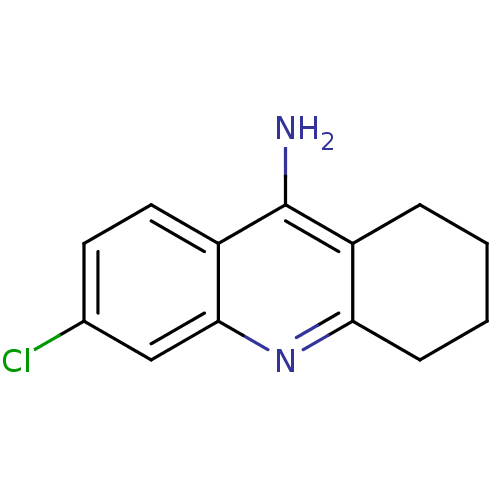 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.06 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50280625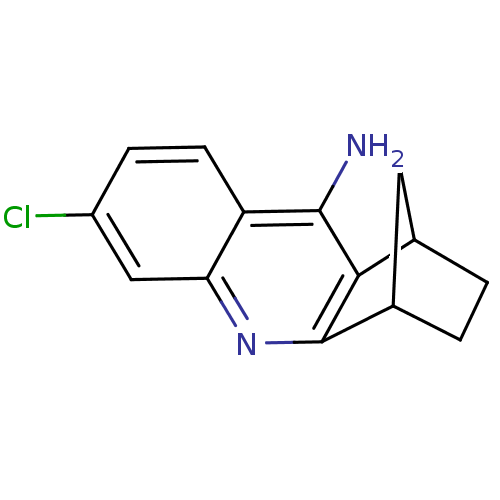 (7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130921 (US8822500, [24A]) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.53 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130919 (US8822500, [18]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50279984 (8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50280625 (7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130912 (US8822500, [1]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.00 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.98 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130921 (US8822500, [24A]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.99 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130913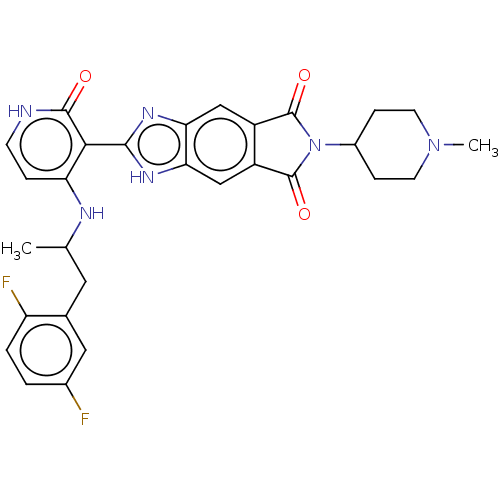 (US8822500, [28]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130915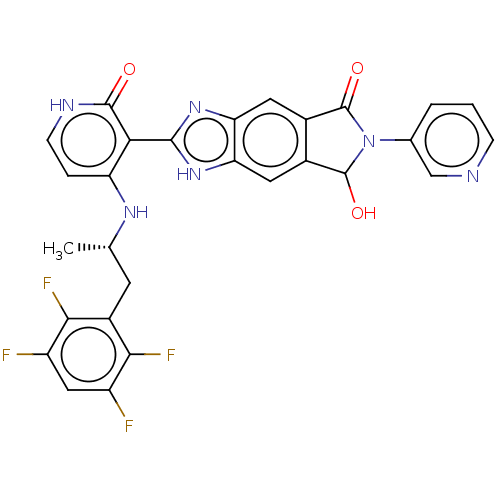 (US8822500, [13]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50280627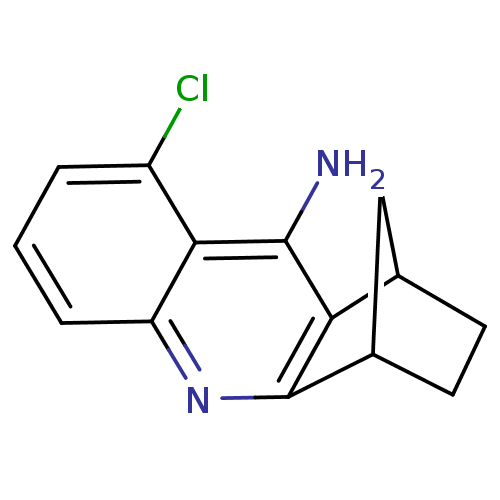 (5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130919 (US8822500, [18]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.16 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50279984 (8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130910 (US8822500, [4]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.81 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130920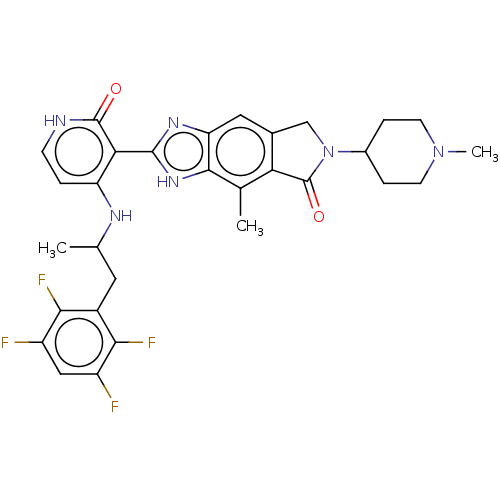 (US8822500, [24]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130921 (US8822500, [24A]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50060470 (10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130911 (US8822500, [2]) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50279984 (8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130919 (US8822500, [18]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM130911 (US8822500, [2]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.0 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130911 (US8822500, [2]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.0 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130921 (US8822500, [24A]) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50280627 (5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |