

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271367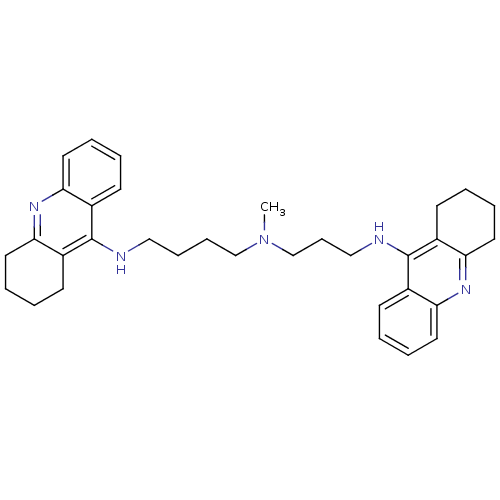 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50005193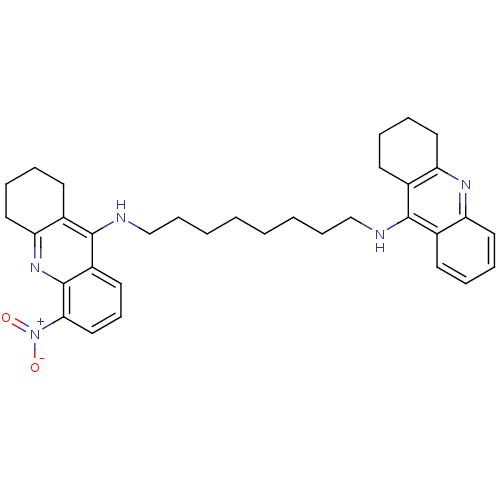 (CHEMBL3099496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005192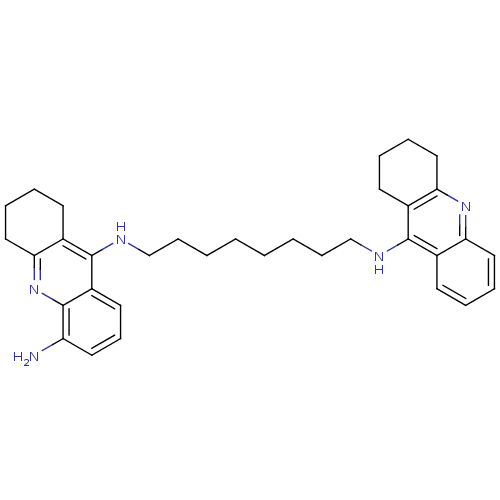 (CHEMBL3099497) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005193 (CHEMBL3099496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50271367 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322373 (1-(3-chlorophenyl)-4-(6-(4-(3-methoxyphenyl)pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50322374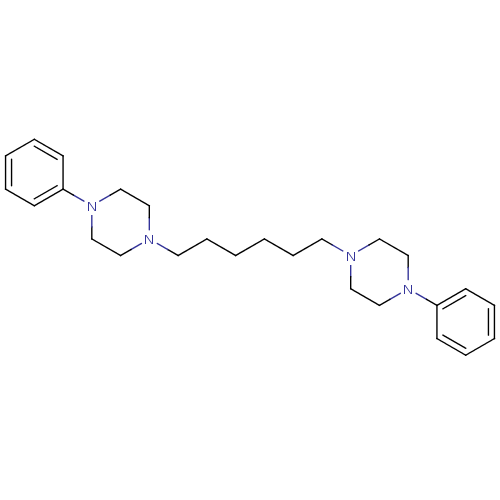 (1,6-bis(4-phenylpiperazin-1-yl)hexane | CHEMBL1172...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265253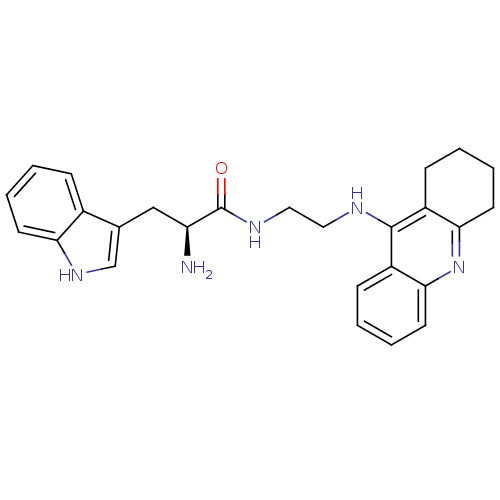 ((S)-2-amino-3-(1H-indol-3-yl)-N-(2-(1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005188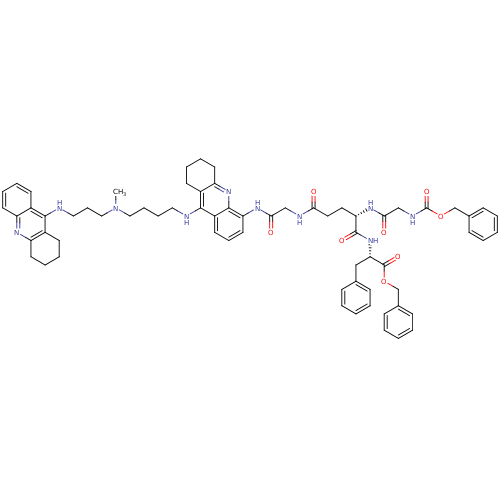 (CHEMBL3099500) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8971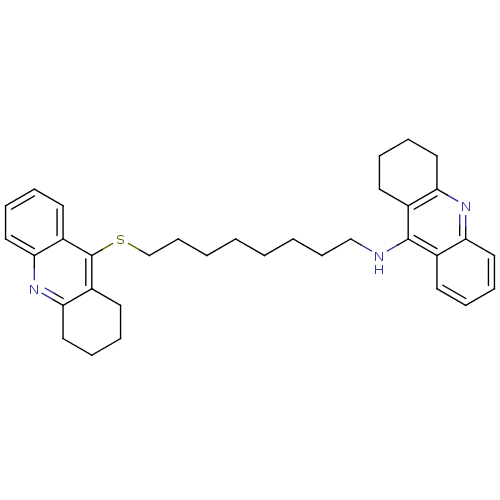 (CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8971 (CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265256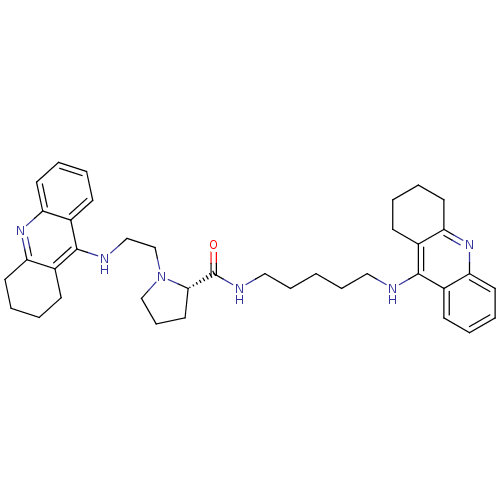 ((S)-1-(2-(1,2,3,4-tetrahydroacridin-9-ylamino)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265252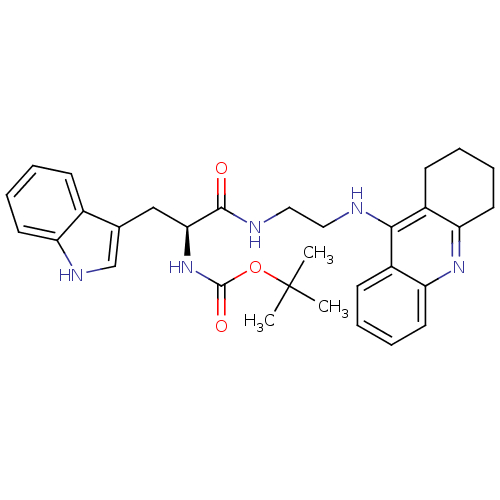 ((S)-tert-butyl 3-(1H-indol-3-yl)-1-oxo-1-(2-(1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005191 (CHEMBL3099498) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322371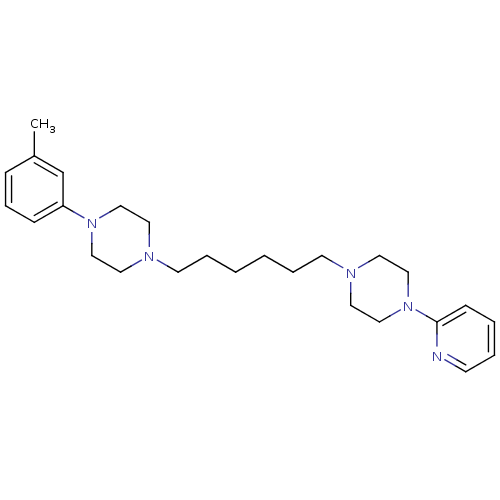 (1-(pyridin-2-yl)-4-(6-(4-m-tolylpiperazin-1-yl)hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50060627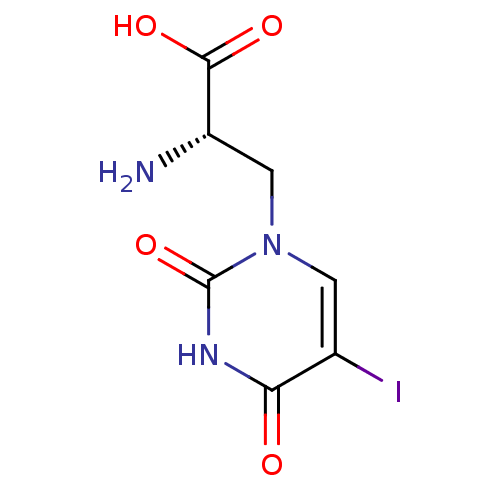 ((S)-2-Amino-3-(5-iodo-2,4-dioxo-3,4-dihydro-2H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat recombinant iGluR5(Q)1b expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50060635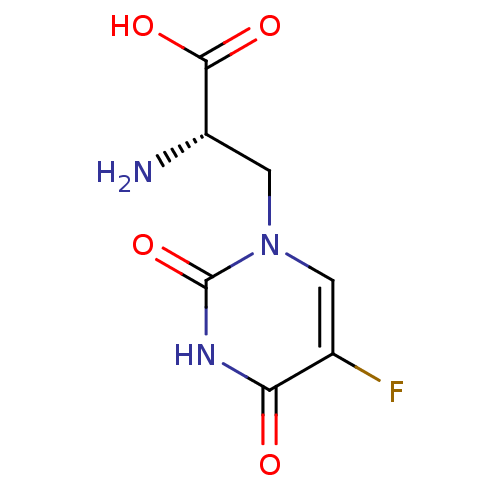 ((S)-2-Amino-3-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of (R,S)-[5-methyl-3H]AMPA from rat recombinant flop iGluR1 expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322374 (1,6-bis(4-phenylpiperazin-1-yl)hexane | CHEMBL1172...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50349983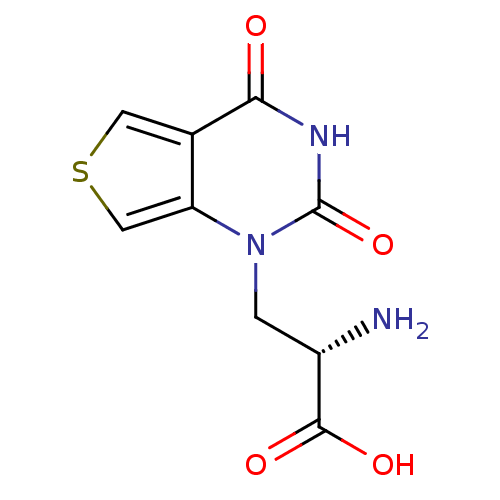 (CHEMBL1812596) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat GluK1 expressed in Sf9 cells | J Med Chem 54: 4793-805 (2011) Article DOI: 10.1021/jm2004078 BindingDB Entry DOI: 10.7270/Q2RR1ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50060635 ((S)-2-Amino-3-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of (R,S)-[5-methyl-3H]AMPA from rat recombinant flop iGluR2(R) expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50322373 (1-(3-chlorophenyl)-4-(6-(4-(3-methoxyphenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50265376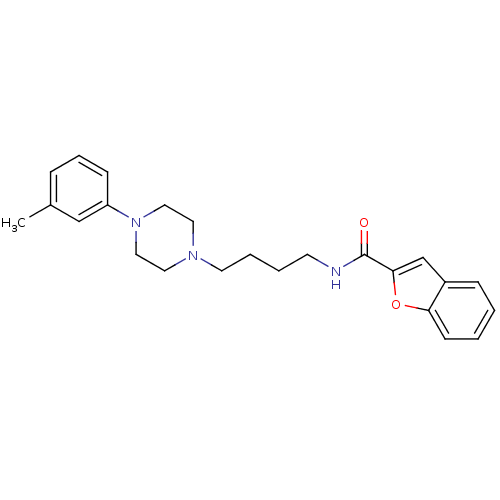 (CHEMBL496739 | N-(4-(4-(m-Tolyl)piperazin-1-yl)but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005189 (CHEMBL3099499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50252920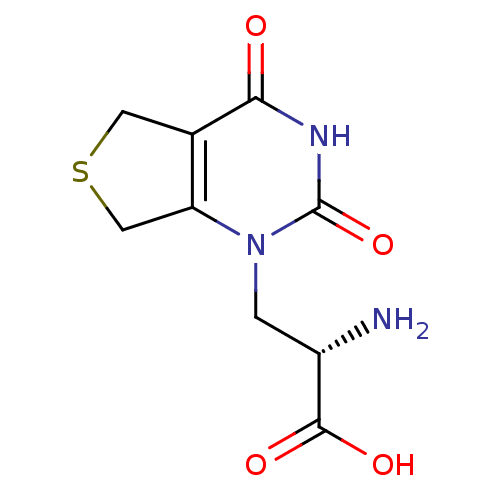 ((S)-1-[2'-Amino-2'-carboxyethyl]-5,7-dihydrothieno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat GluK1 expressed in Sf9 cells | J Med Chem 54: 4793-805 (2011) Article DOI: 10.1021/jm2004078 BindingDB Entry DOI: 10.7270/Q2RR1ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50252922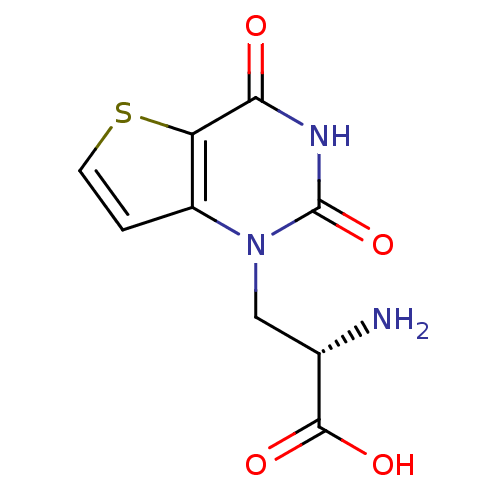 ((S)-1-(2'-Amino-2'-carboxyethyl)thieno[3,2-d]pyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat GluK1 expressed in Sf9 cells | J Med Chem 54: 4793-805 (2011) Article DOI: 10.1021/jm2004078 BindingDB Entry DOI: 10.7270/Q2RR1ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50349981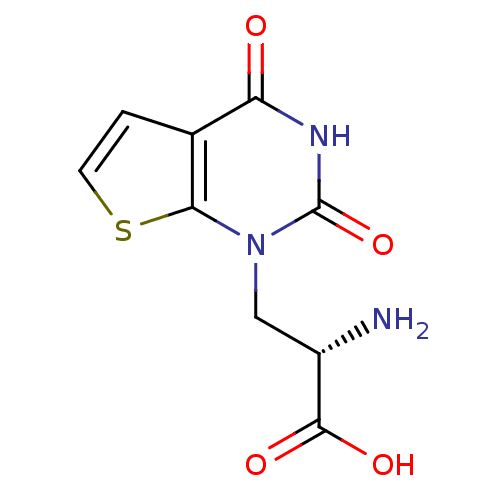 (CHEMBL1812598) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat GluK1 expressed in Sf9 cells | J Med Chem 54: 4793-805 (2011) Article DOI: 10.1021/jm2004078 BindingDB Entry DOI: 10.7270/Q2RR1ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50322372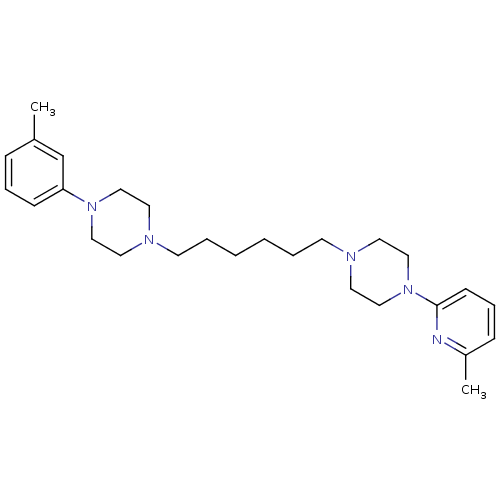 (1-(6-methylpyridin-2-yl)-4-(6-(4-m-tolylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50265255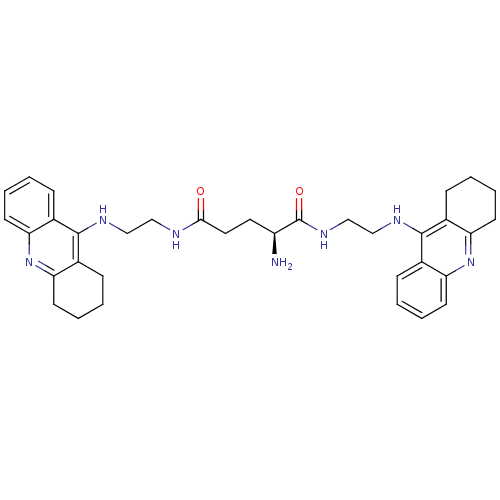 ((S)-2-amino-N1,N5-bis(2-(1,2,3,4-tetrahydroacridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50265256 ((S)-1-(2-(1,2,3,4-tetrahydroacridin-9-ylamino)ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50322373 (1-(3-chlorophenyl)-4-(6-(4-(3-methoxyphenyl)pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322376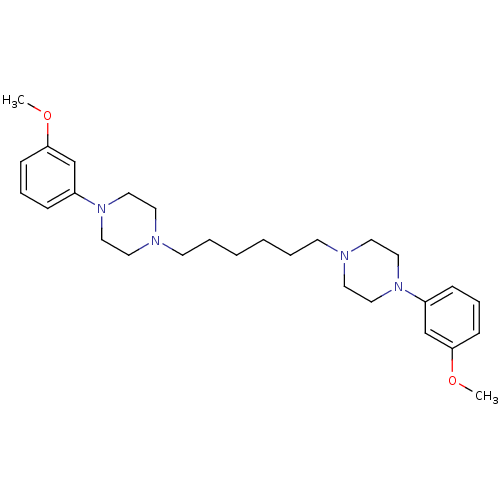 (1,6-bis(4-(3-methoxyphenyl)piperazin-1-yl)hexane |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322380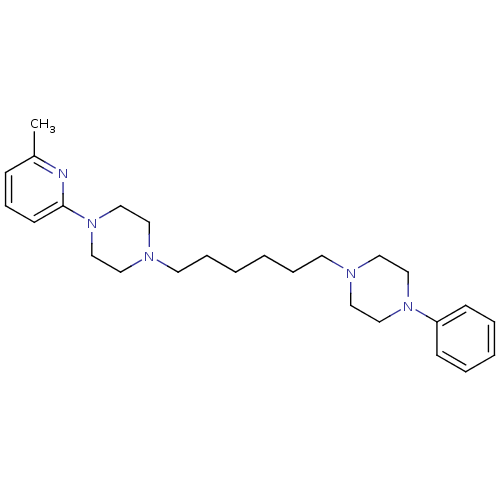 (1-(6-methylpyridin-2-yl)-4-(6-(4-phenylpiperazin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50005192 (CHEMBL3099497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50322369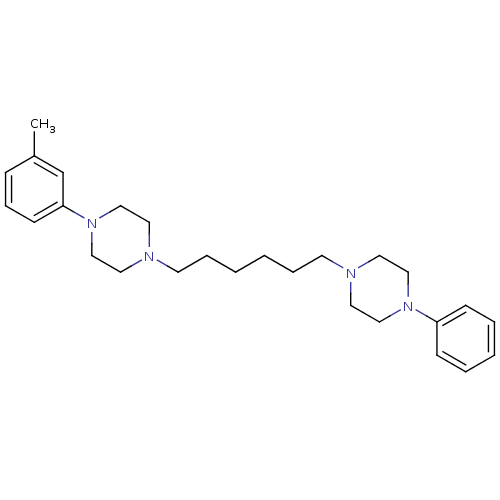 (1-phenyl-4-(6-(4-m-tolylpiperazin-1-yl)hexyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50322371 (1-(pyridin-2-yl)-4-(6-(4-m-tolylpiperazin-1-yl)hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50322372 (1-(6-methylpyridin-2-yl)-4-(6-(4-m-tolylpiperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50322377 (1,6-bis(4-(3-chlorophenyl)piperazin-1-yl)hexane | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in CRL:CD(SD)BR-COBS rat striatum by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50265376 (CHEMBL496739 | N-(4-(4-(m-Tolyl)piperazin-1-yl)but...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]8OH-DPAT from 5HT1A receptor in rat CRL:CD(SD)BR-COBS hippocampus by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265254 ((S)-tert-butyl 1,5-dioxo-1,5-bis(2-(1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Rattus norvegicus) | BDBM50002369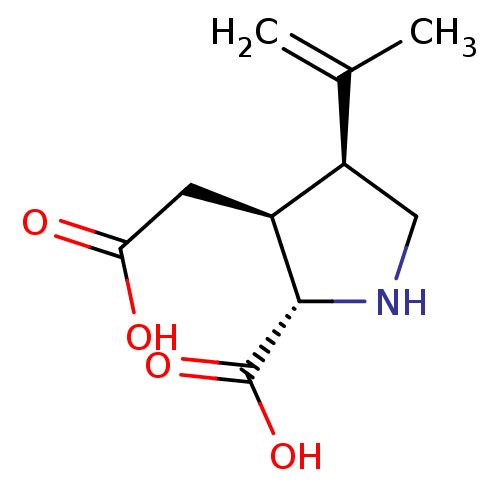 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of [3H]kainic acid from rat recombinant iGluR6(V,C,R) receptor expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50252873 (2-amino-3-(5-bromo-2,4-dioxo-3,4-dihydropyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat recombinant iGluR5(Q)1b expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50265376 (CHEMBL496739 | N-(4-(4-(m-Tolyl)piperazin-1-yl)but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 53: 4803-7 (2010) Article DOI: 10.1021/jm100294b BindingDB Entry DOI: 10.7270/Q28P60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50265254 ((S)-tert-butyl 1,5-dioxo-1,5-bis(2-(1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | Bioorg Med Chem Lett 18: 5213-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.076 BindingDB Entry DOI: 10.7270/Q29W0F9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 325 total ) | Next | Last >> |