 Found 374 hits with Last Name = 'guzi' and Initial = 'tj'
Found 374 hits with Last Name = 'guzi' and Initial = 'tj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598680
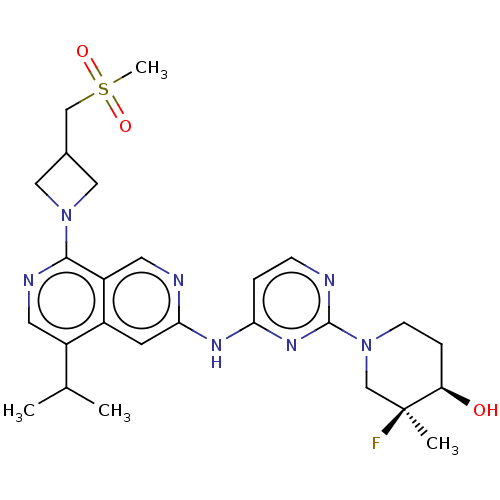
(CHEMBL5173517)Show SMILES CC(C)c1cnc(N2CC(CS(C)(=O)=O)C2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598682
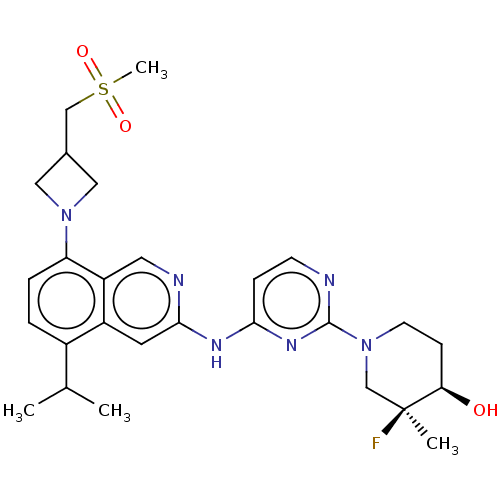
(CHEMBL5206444)Show SMILES CC(C)c1ccc(N2CC(CS(C)(=O)=O)C2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598681
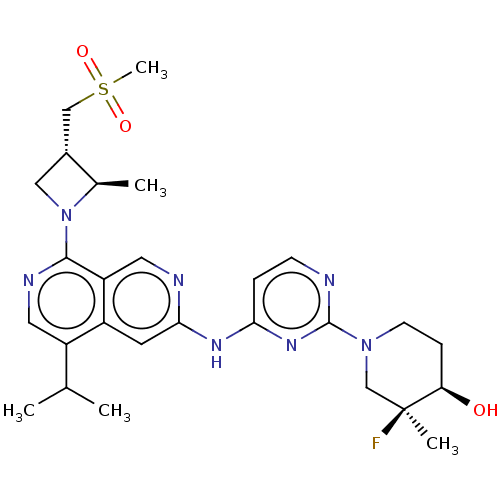
(CHEMBL5200991)Show SMILES CC(C)c1cnc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598673
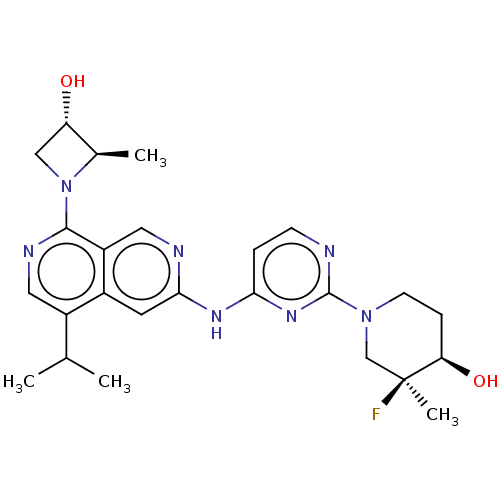
(CHEMBL5207390)Show SMILES CC(C)c1cnc(N2C[C@H](O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598683
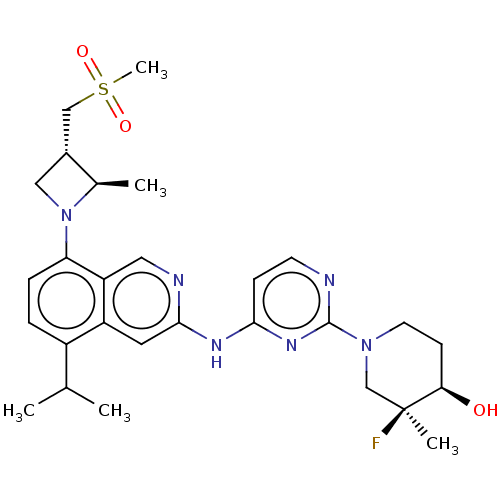
(CHEMBL5196495)Show SMILES CC(C)c1ccc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598668
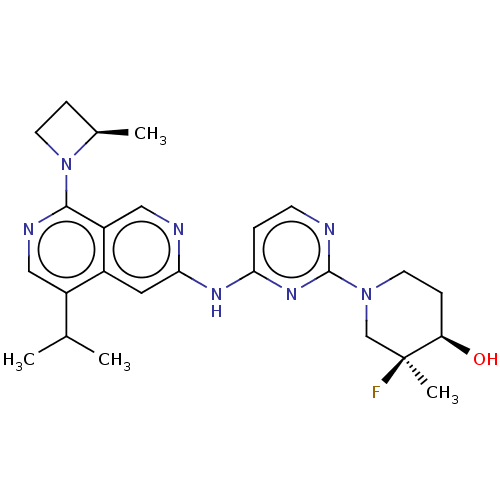
(CHEMBL5195164)Show SMILES CC(C)c1cnc(N2CC[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598685
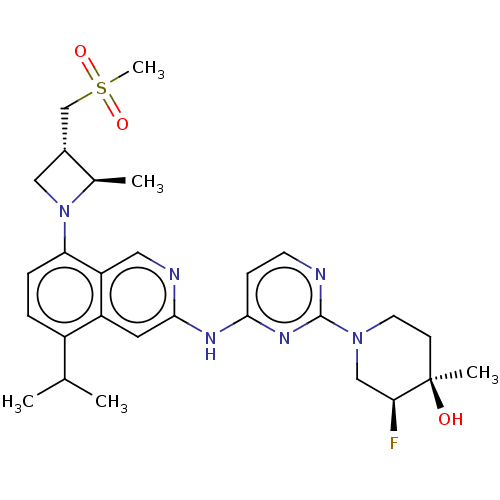
(CHEMBL5208737)Show SMILES CC(C)c1ccc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@](C)(O)[C@@H](F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598686
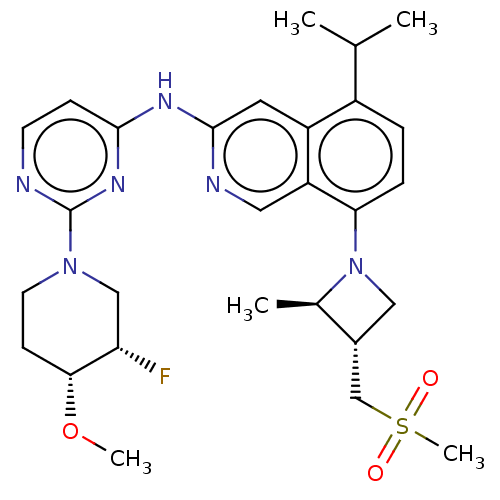
(BLU-945 | BLU945 | Blu-945)Show SMILES CO[C@@H]1CCN(C[C@@H]1F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598684
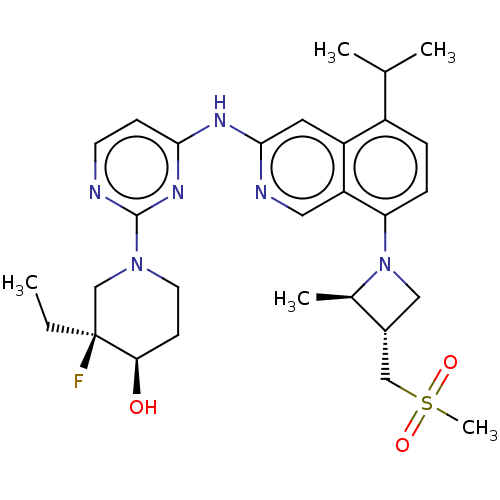
(CHEMBL5194722)Show SMILES CC[C@]1(F)CN(CC[C@H]1O)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598675

(CHEMBL5185207)Show SMILES CC(C)c1ccc(N2C[C@H](O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598686

(BLU-945 | BLU945 | Blu-945)Show SMILES CO[C@@H]1CCN(C[C@@H]1F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598687
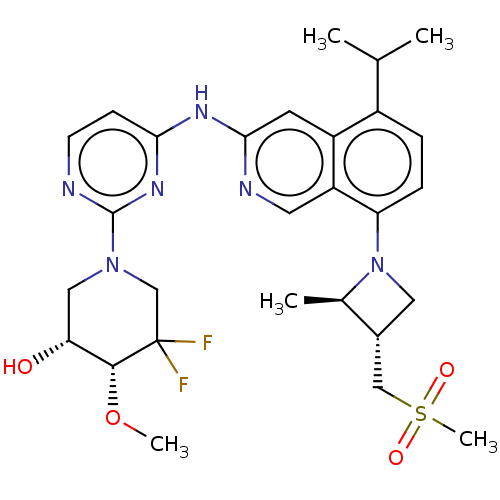
(CHEMBL5193016)Show SMILES CO[C@@H]1[C@H](O)CN(CC1(F)F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598686

(BLU-945 | BLU945 | Blu-945)Show SMILES CO[C@@H]1CCN(C[C@@H]1F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598679
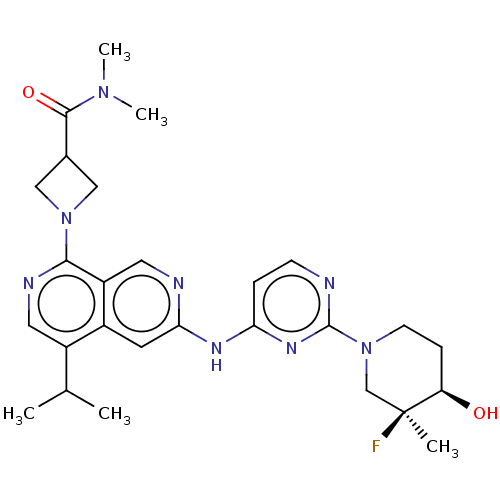
(CHEMBL5170376)Show SMILES CC(C)c1cnc(N2CC(C2)C(=O)N(C)C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598683

(CHEMBL5196495)Show SMILES CC(C)c1ccc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598678

(CHEMBL5187021)Show SMILES CNC(=O)C1CN(C1)c1ncc(C(C)C)c2cc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)ncc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598686

(BLU-945 | BLU945 | Blu-945)Show SMILES CO[C@@H]1CCN(C[C@@H]1F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442673
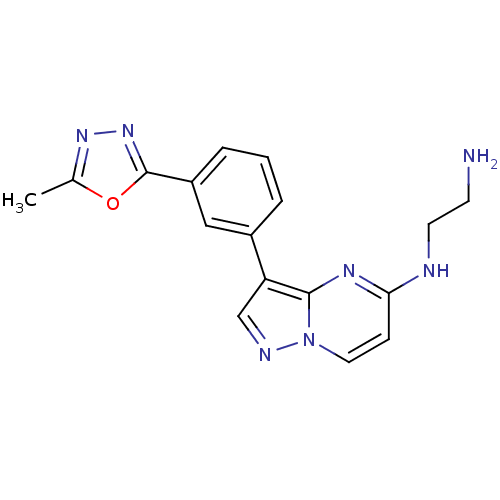
(CHEMBL2442291)Show InChI InChI=1S/C17H17N7O/c1-11-22-23-17(25-11)13-4-2-3-12(9-13)14-10-20-24-8-5-15(19-7-6-18)21-16(14)24/h2-5,8-10H,6-7,18H2,1H3,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442690
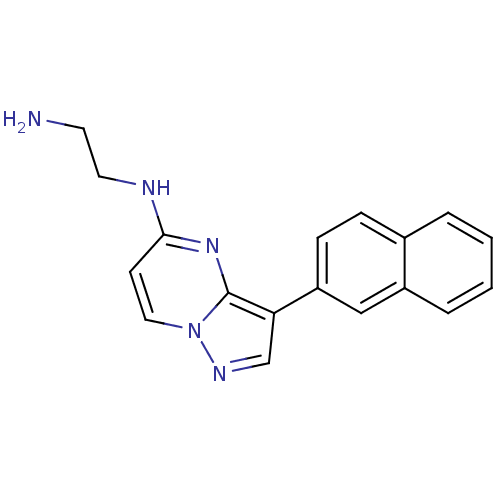
(CHEMBL2442296)Show InChI InChI=1S/C18H17N5/c19-8-9-20-17-7-10-23-18(22-17)16(12-21-23)15-6-5-13-3-1-2-4-14(13)11-15/h1-7,10-12H,8-9,19H2,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442685
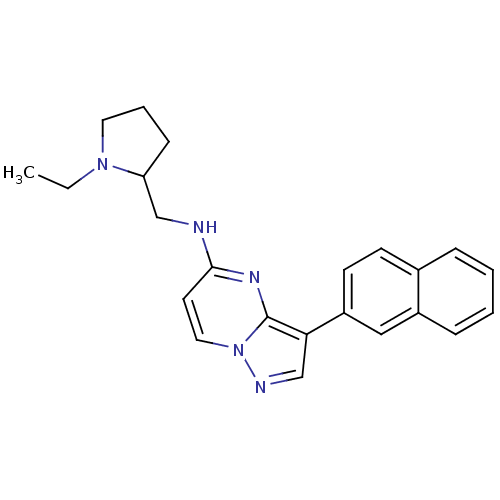
(CHEMBL2442301)Show InChI InChI=1S/C23H25N5/c1-2-27-12-5-8-20(27)15-24-22-11-13-28-23(26-22)21(16-25-28)19-10-9-17-6-3-4-7-18(17)14-19/h3-4,6-7,9-11,13-14,16,20H,2,5,8,12,15H2,1H3,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598681

(CHEMBL5200991)Show SMILES CC(C)c1cnc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598684

(CHEMBL5194722)Show SMILES CC[C@]1(F)CN(CC[C@H]1O)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50442673

(CHEMBL2442291)Show InChI InChI=1S/C17H17N7O/c1-11-22-23-17(25-11)13-4-2-3-12(9-13)14-10-20-24-8-5-15(19-7-6-18)21-16(14)24/h2-5,8-10H,6-7,18H2,1H3,(H,19,21) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598674
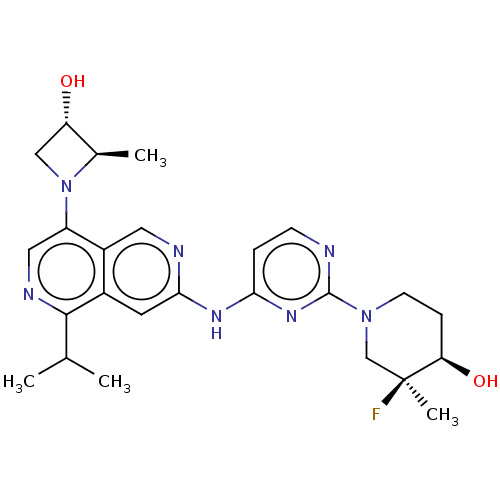
(CHEMBL5172587)Show SMILES CC(C)c1ncc(N2C[C@H](O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442679

(CHEMBL2442302)Show InChI InChI=1S/C16H15N5S/c17-5-6-18-15-3-7-21-16(20-15)13(10-19-21)11-1-2-14-12(9-11)4-8-22-14/h1-4,7-10H,5-6,17H2,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598667
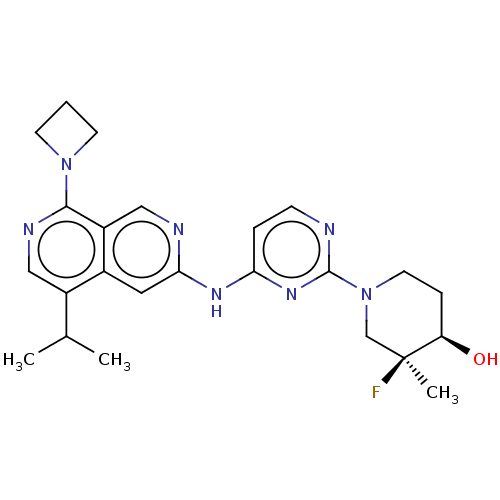
(CHEMBL5208679)Show SMILES CC(C)c1cnc(N2CCC2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598671
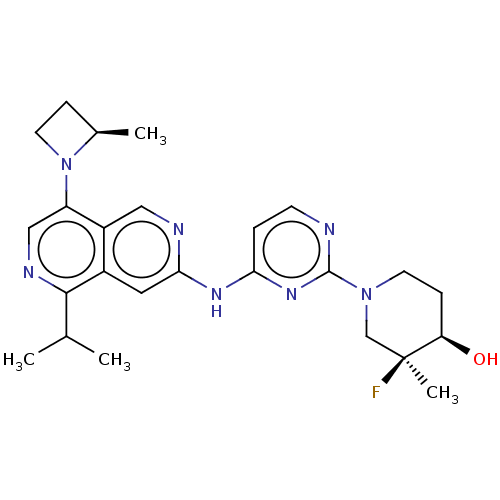
(CHEMBL5182741)Show SMILES CC(C)c1ncc(N2CC[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598687

(CHEMBL5193016)Show SMILES CO[C@@H]1[C@H](O)CN(CC1(F)F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598682

(CHEMBL5206444)Show SMILES CC(C)c1ccc(N2CC(CS(C)(=O)=O)C2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442692
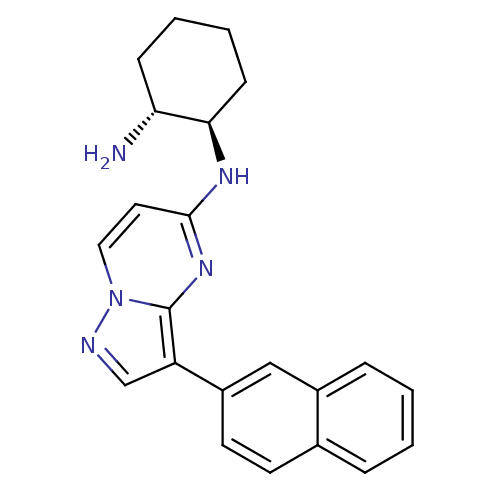
(CHEMBL2442317)Show SMILES N[C@@H]1CCCC[C@H]1Nc1ccn2ncc(-c3ccc4ccccc4c3)c2n1 |r| Show InChI InChI=1S/C22H23N5/c23-19-7-3-4-8-20(19)25-21-11-12-27-22(26-21)18(14-24-27)17-10-9-15-5-1-2-6-16(15)13-17/h1-2,5-6,9-14,19-20H,3-4,7-8,23H2,(H,25,26)/t19-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334853
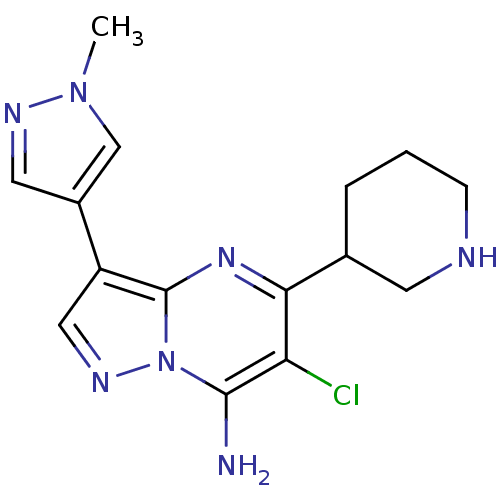
(6-chloro-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin...)Show InChI InChI=1S/C15H18ClN7/c1-22-8-10(6-19-22)11-7-20-23-14(17)12(16)13(21-15(11)23)9-3-2-4-18-5-9/h6-9,18H,2-5,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334854
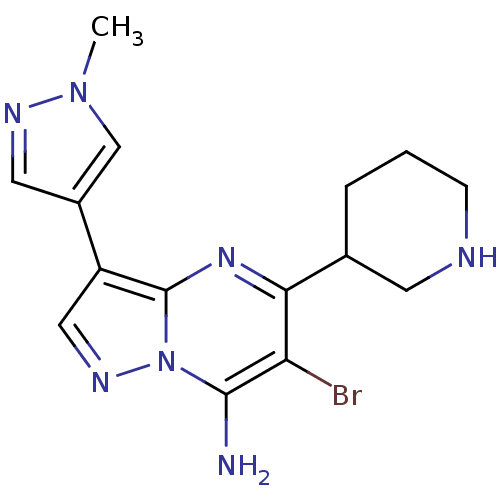
(6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-...)Show InChI InChI=1S/C15H18BrN7/c1-22-8-10(6-19-22)11-7-20-23-14(17)12(16)13(21-15(11)23)9-3-2-4-18-5-9/h6-9,18H,2-5,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334855

(6-iodo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-3...)Show InChI InChI=1S/C15H18IN7/c1-22-8-10(6-19-22)11-7-20-23-14(17)12(16)13(21-15(11)23)9-3-2-4-18-5-9/h6-9,18H,2-5,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50223567

(3-bromo-5-(2-chlorophenyl)-N-(pyridin-3-ylmethyl)p...)Show InChI InChI=1S/C18H13BrClN5/c19-14-11-23-25-17(22-10-12-4-3-7-21-9-12)8-16(24-18(14)25)13-5-1-2-6-15(13)20/h1-9,11,22H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). |
US Patent US8580782 (2013)
BindingDB Entry DOI: 10.7270/Q2VM49WG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442693

(CHEMBL2442316)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ccn2ncc(-c3ccc4ccccc4c3)c2n1 |r,wU:1.0,wD:4.7,(62.54,-18.45,;63.88,-17.68,;63.89,-16.14,;65.21,-15.38,;66.54,-16.16,;66.55,-17.69,;65.21,-18.46,;67.88,-15.39,;69.21,-16.16,;69.21,-17.7,;70.54,-18.47,;71.87,-17.7,;73.35,-18.18,;74.26,-16.93,;73.35,-15.67,;73.83,-14.21,;72.8,-13.07,;73.27,-11.61,;74.78,-11.28,;75.24,-9.82,;76.76,-9.51,;77.8,-10.65,;77.32,-12.12,;75.81,-12.43,;75.34,-13.89,;71.87,-16.15,;70.54,-15.39,)| Show InChI InChI=1S/C22H23N5/c23-18-7-9-19(10-8-18)25-21-11-12-27-22(26-21)20(14-24-27)17-6-5-15-3-1-2-4-16(15)13-17/h1-6,11-14,18-19H,7-10,23H2,(H,25,26)/t18-,19- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598686

(BLU-945 | BLU945 | Blu-945)Show SMILES CO[C@@H]1CCN(C[C@@H]1F)c1nccc(Nc2cc3c(ccc(N4C[C@H](CS(C)(=O)=O)[C@H]4C)c3cn2)C(C)C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598676
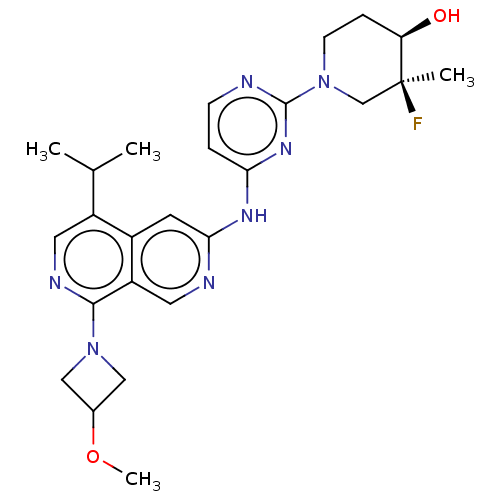
(CHEMBL5205583)Show SMILES COC1CN(C1)c1ncc(C(C)C)c2cc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)ncc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598672
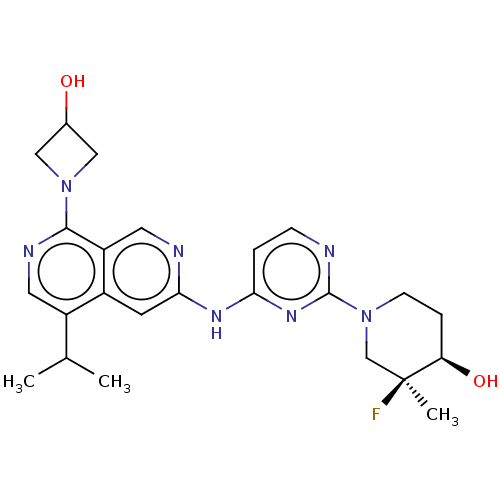
(CHEMBL5203267)Show SMILES CC(C)c1cnc(N2CC(O)C2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598685

(CHEMBL5208737)Show SMILES CC(C)c1ccc(N2C[C@H](CS(C)(=O)=O)[C@H]2C)c2cnc(Nc3ccnc(n3)N3CC[C@@](C)(O)[C@@H](F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442681

(CHEMBL2442290)Show InChI InChI=1S/C18H17N5O/c19-7-8-20-17-6-9-23-18(22-17)15(12-21-23)13-3-1-4-14(11-13)16-5-2-10-24-16/h1-6,9-12H,7-8,19H2,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50598680

(CHEMBL5173517)Show SMILES CC(C)c1cnc(N2CC(CS(C)(=O)=O)C2)c2cnc(Nc3ccnc(n3)N3CC[C@@H](O)[C@@](C)(F)C3)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00704
BindingDB Entry DOI: 10.7270/Q2RJ4PHG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442686
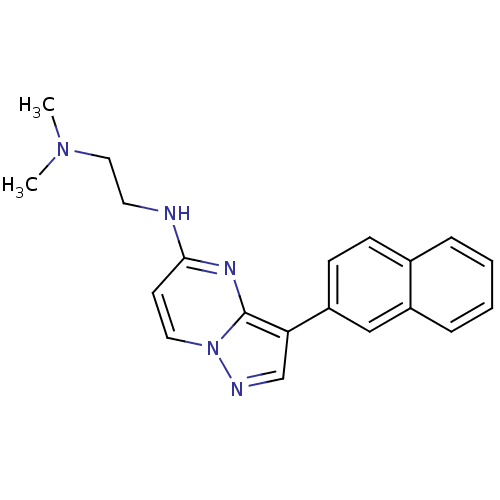
(CHEMBL2442300)Show InChI InChI=1S/C20H21N5/c1-24(2)12-10-21-19-9-11-25-20(23-19)18(14-22-25)17-8-7-15-5-3-4-6-16(15)13-17/h3-9,11,13-14H,10,12H2,1-2H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334849
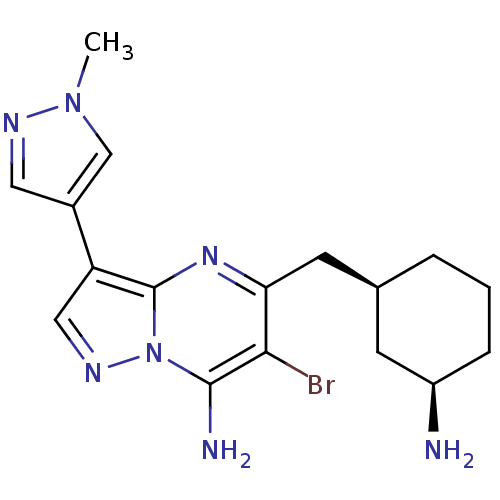
(CHEMBL1643236 | Syn-5-((3-aminocyclohexyl)methyl)-...)Show SMILES Cn1cc(cn1)-c1cnn2c(N)c(Br)c(C[C@H]3CCC[C@@H](N)C3)nc12 |r| Show InChI InChI=1S/C17H22BrN7/c1-24-9-11(7-21-24)13-8-22-25-16(20)15(18)14(23-17(13)25)6-10-3-2-4-12(19)5-10/h7-10,12H,2-6,19-20H2,1H3/t10-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442687
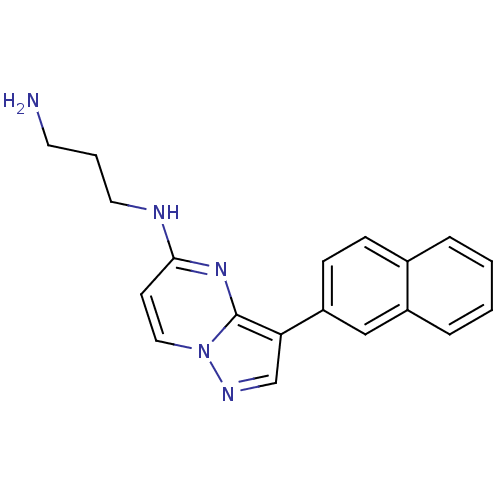
(CHEMBL2442299)Show InChI InChI=1S/C19H19N5/c20-9-3-10-21-18-8-11-24-19(23-18)17(13-22-24)16-7-6-14-4-1-2-5-15(14)12-16/h1-2,4-8,11-13H,3,9-10,20H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM105239

(US8580782, 7)Show InChI InChI=1S/C18H13BrClN5/c19-14-11-23-25-17(22-10-12-5-7-21-8-6-12)9-16(24-18(14)25)13-3-1-2-4-15(13)20/h1-9,11,22H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). |
US Patent US8580782 (2013)
BindingDB Entry DOI: 10.7270/Q2VM49WG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334876
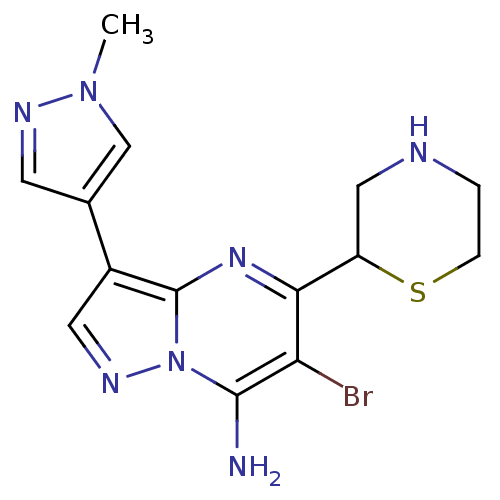
(6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(thiomorpho...)Show InChI InChI=1S/C14H16BrN7S/c1-21-7-8(4-18-21)9-5-19-22-13(16)11(15)12(20-14(9)22)10-6-17-2-3-23-10/h4-5,7,10,17H,2-3,6,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442691

(CHEMBL2442295)Show InChI InChI=1S/C20H19N5/c1-2-4-16-13-17(6-5-15(16)3-1)18-14-22-25-10-7-19(23-20(18)25)24-11-8-21-9-12-24/h1-7,10,13-14,21H,8-9,11-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442698

(CHEMBL2442287)Show InChI InChI=1S/C13H17N7/c1-19(2)6-4-14-12-3-5-20-13(18-12)11(9-17-20)10-7-15-16-8-10/h3,5,7-9H,4,6H2,1-2H3,(H,14,18)(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50334847
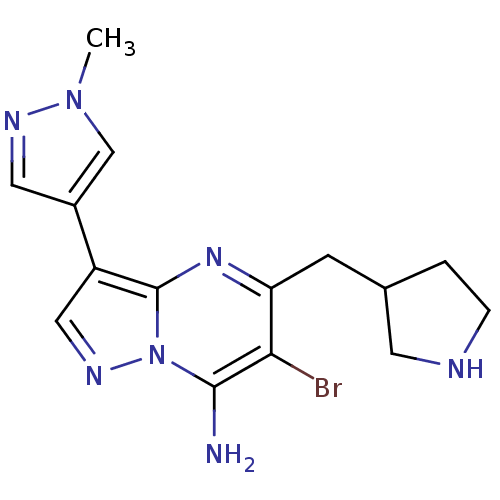
(6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(pyrrolidin...)Show InChI InChI=1S/C15H18BrN7/c1-22-8-10(6-19-22)11-7-20-23-14(17)13(16)12(21-15(11)23)4-9-2-3-18-5-9/h6-9,18H,2-5,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 21: 471-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.114
BindingDB Entry DOI: 10.7270/Q2P26ZC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50442678
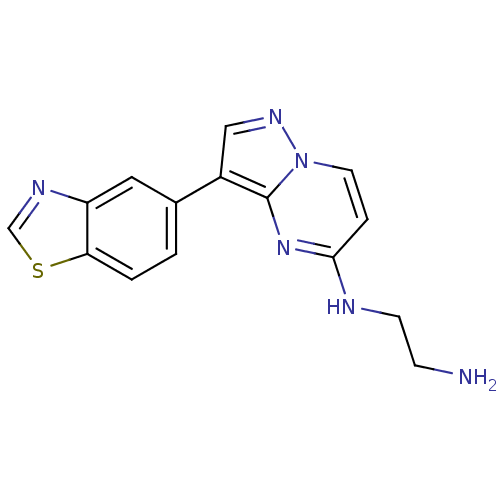
(CHEMBL2442303)Show InChI InChI=1S/C15H14N6S/c16-4-5-17-14-3-6-21-15(20-14)11(8-19-21)10-1-2-13-12(7-10)18-9-22-13/h1-3,6-9H,4-5,16H2,(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... |
Bioorg Med Chem Lett 23: 6178-82 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.110
BindingDB Entry DOI: 10.7270/Q2MS3V69 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data