 Found 19 hits with Last Name = 'habeck' and Initial = 'll'
Found 19 hits with Last Name = 'habeck' and Initial = 'll' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288820
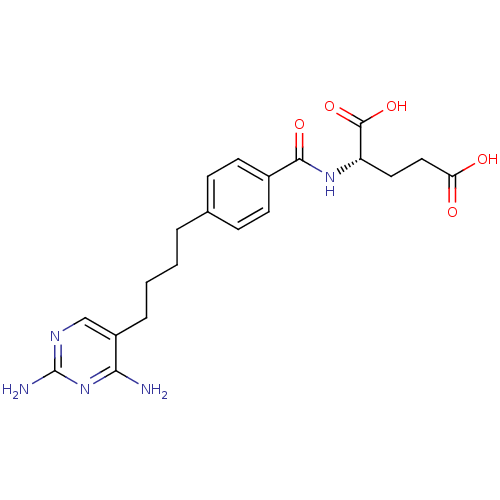
((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073754
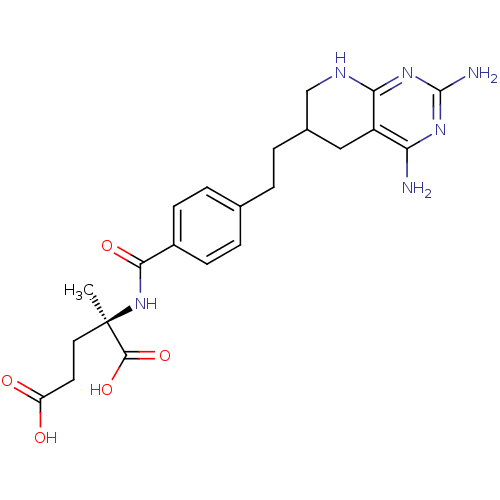
((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073752
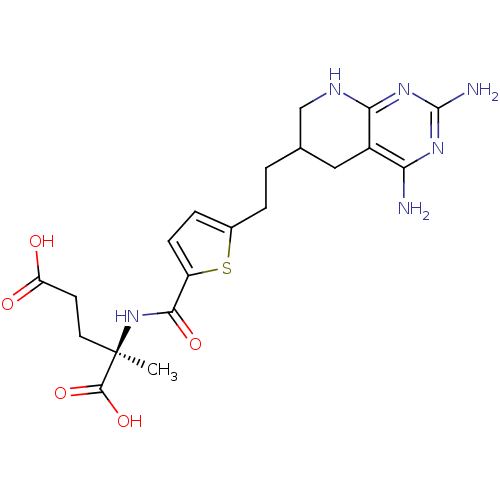
((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM22590
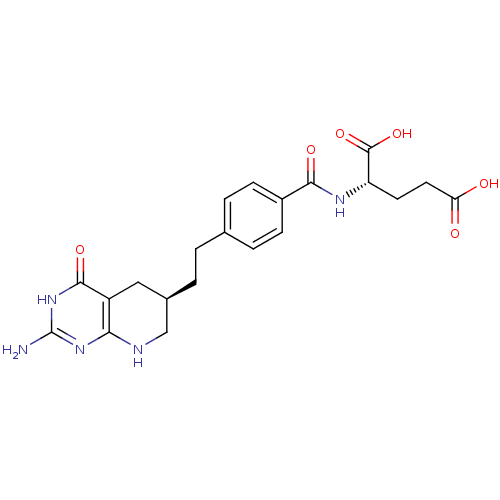
((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...)Show SMILES Nc1nc2NC[C@H](CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C21H25N5O6/c22-21-25-17-14(19(30)26-21)9-12(10-23-17)2-1-11-3-5-13(6-4-11)18(29)24-15(20(31)32)7-8-16(27)28/h3-6,12,15H,1-2,7-10H2,(H,24,29)(H,27,28)(H,31,32)(H4,22,23,25,26,30)/t12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of trifunctional Glycinamide ribonucleotide formyltransferase isolated from murine L1210 cells. |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM22590

((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...)Show SMILES Nc1nc2NC[C@H](CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C21H25N5O6/c22-21-25-17-14(19(30)26-21)9-12(10-23-17)2-1-11-3-5-13(6-4-11)18(29)24-15(20(31)32)7-8-16(27)28/h3-6,12,15H,1-2,7-10H2,(H,24,29)(H,27,28)(H,31,32)(H4,22,23,25,26,30)/t12-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of recombinant human monofunctional Glycinamide ribonucleotide formyltransferase |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073753

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)o1)C(O)=O Show InChI InChI=1S/C20H26N6O6/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073752

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50073754

((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Thymidylate synthase |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human thymidylate synthase (TS) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50288820

((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human thymidylate synthase (TS). |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073752

((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50281128
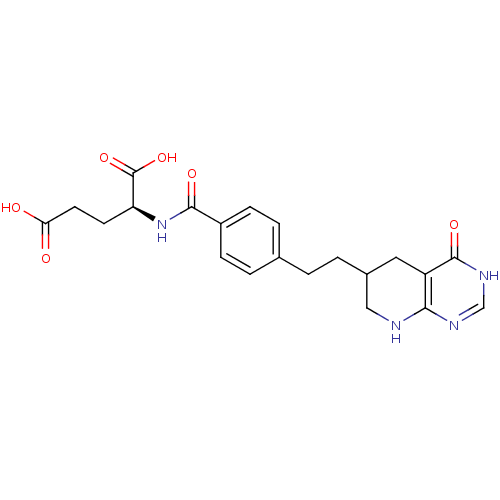
((S)-2-{4-[2-(4-Oxo-3,4,5,6,7,8-hexahydro-pyrido[2,...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(CCC2CNc3nc[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C21H24N4O6/c26-17(27)8-7-16(21(30)31)25-19(28)14-5-3-12(4-6-14)1-2-13-9-15-18(22-10-13)23-11-24-20(15)29/h3-6,11,13,16H,1-2,7-10H2,(H,25,28)(H,26,27)(H,30,31)(H2,22,23,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of trifunctional Glycinamide ribonucleotide formyltransferase isolated from murine L1210 cells. |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50073754

((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50281128

((S)-2-{4-[2-(4-Oxo-3,4,5,6,7,8-hexahydro-pyrido[2,...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(CCC2CNc3nc[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C21H24N4O6/c26-17(27)8-7-16(21(30)31)25-19(28)14-5-3-12(4-6-14)1-2-13-9-15-18(22-10-13)23-11-24-20(15)29/h3-6,11,13,16H,1-2,7-10H2,(H,25,28)(H,26,27)(H,30,31)(H2,22,23,24,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of recombinant human monofunctional Glycinamide ribonucleotide formyltransferase |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50281129
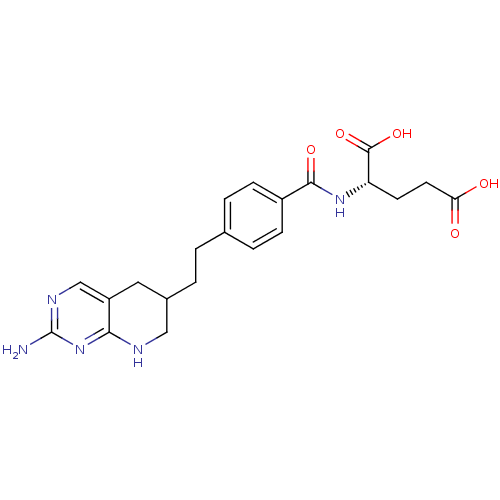
((S)-2-{4-[2-(2-Amino-5,6,7,8-tetrahydro-pyrido[2,3...)Show SMILES Nc1ncc2CC(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)CNc2n1 Show InChI InChI=1S/C21H25N5O5/c22-21-24-11-15-9-13(10-23-18(15)26-21)2-1-12-3-5-14(6-4-12)19(29)25-16(20(30)31)7-8-17(27)28/h3-6,11,13,16H,1-2,7-10H2,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of recombinant human monofunctional Glycinamide ribonucleotide formyltransferase |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50281129

((S)-2-{4-[2-(2-Amino-5,6,7,8-tetrahydro-pyrido[2,3...)Show SMILES Nc1ncc2CC(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)CNc2n1 Show InChI InChI=1S/C21H25N5O5/c22-21-24-11-15-9-13(10-23-18(15)26-21)2-1-12-3-5-14(6-4-12)19(29)25-16(20(30)31)7-8-17(27)28/h3-6,11,13,16H,1-2,7-10H2,(H,25,29)(H,27,28)(H,30,31)(H3,22,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition against the human dihydrofolate reductase |
Bioorg Med Chem Lett 3: 2657-2660 (1993)
Article DOI: 10.1016/S0960-894X(01)80736-8
BindingDB Entry DOI: 10.7270/Q2R49QPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data