 Found 87 hits with Last Name = 'haider' and Initial = 'mr'
Found 87 hits with Last Name = 'haider' and Initial = 'mr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50445253

(CHEMBL1232500)Show InChI InChI=1S/C12H13NO2S/c1-3-13-10-7-9(15)4-5-11(10)16-12(13)6-8(2)14/h4-7,15H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50041419
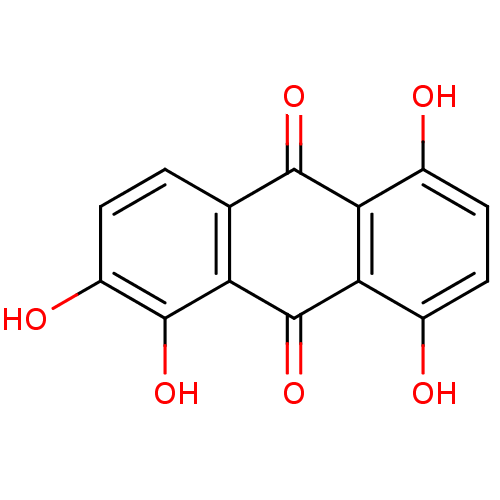
(1,2,5,8-tetrahydroxy-9,10-anthracenedione | 1,2,5,...)Show InChI InChI=1S/C14H8O6/c15-6-3-4-7(16)11-10(6)12(18)5-1-2-8(17)13(19)9(5)14(11)20/h1-4,15-17,19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50449244
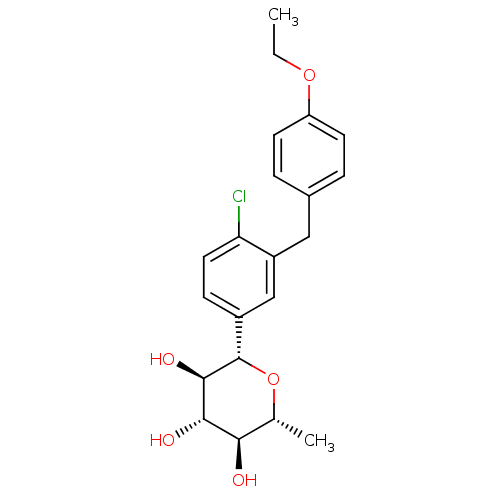
(CHEMBL3125150)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](C)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5/c1-3-26-16-7-4-13(5-8-16)10-15-11-14(6-9-17(15)22)21-20(25)19(24)18(23)12(2)27-21/h4-9,11-12,18-21,23-25H,3,10H2,1-2H3/t12-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222244
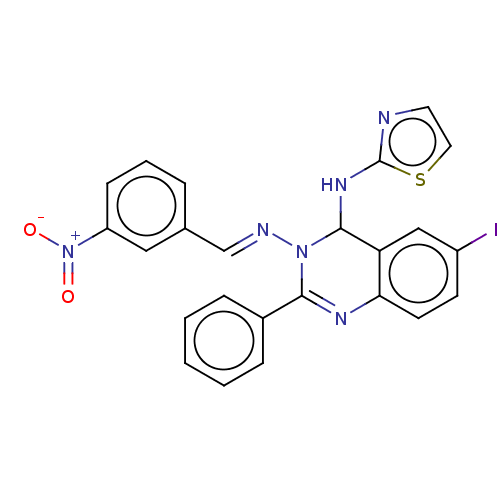
(6-Iodo-N3-(3-nitrobenzylidene)-2-phenyl-N4-(thiazo...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)c1 |c:27| Show InChI InChI=1S/C24H17IN6O2S/c25-18-9-10-21-20(14-18)23(29-24-26-11-12-34-24)30(22(28-21)17-6-2-1-3-7-17)27-15-16-5-4-8-19(13-16)31(32)33/h1-15,23H,(H,26,29)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50342885
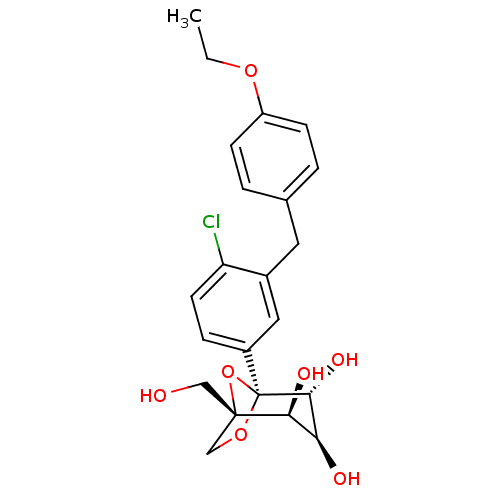
((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phe...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.877 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20880
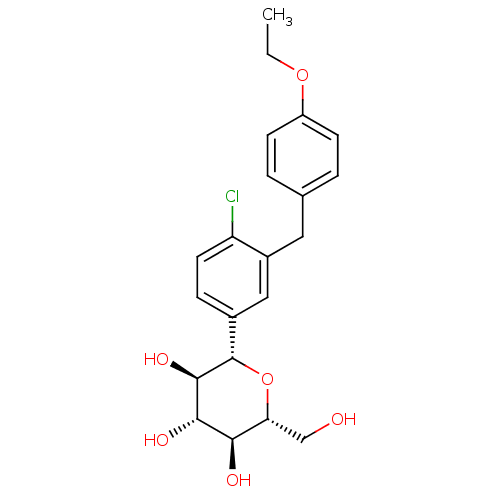
((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222254

(6-Iodo-N4-(4-methylthiazol-2-yl)-N3-(3-nitrobenzyl...)Show SMILES Cc1csc(NC2N(\N=C\c3cccc(c3)[N+]([O-])=O)C(=Nc3ccc(I)cc23)c2ccccc2)n1 |c:20| Show InChI InChI=1S/C25H19IN6O2S/c1-16-15-35-25(28-16)30-24-21-13-19(26)10-11-22(21)29-23(18-7-3-2-4-8-18)31(24)27-14-17-6-5-9-20(12-17)32(33)34/h2-15,24H,1H3,(H,28,30)/b27-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222239
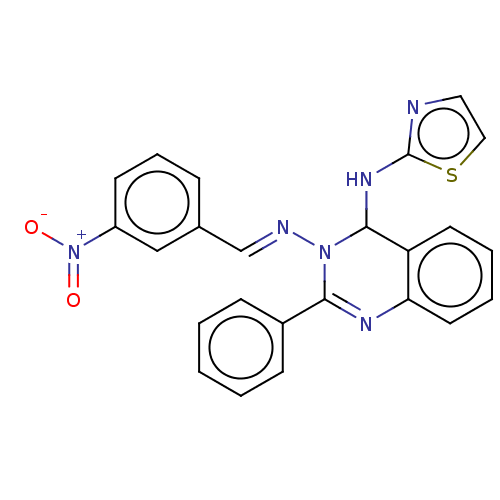
(N3-(3-Nitrobenzylidene)-2-phenyl-N4-(thiazol-2-yl)...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)c1 |c:26| Show InChI InChI=1S/C24H18N6O2S/c31-30(32)19-10-6-7-17(15-19)16-26-29-22(18-8-2-1-3-9-18)27-21-12-5-4-11-20(21)23(29)28-24-25-13-14-33-24/h1-16,23H,(H,25,28)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50235017
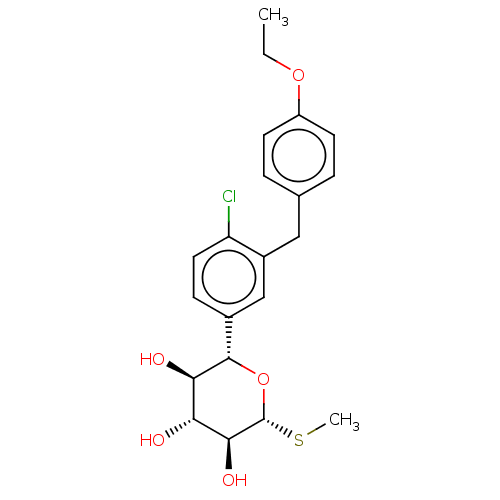
(LP-802034 | LX-4211 | Sotagliflozin)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5S/c1-3-26-15-7-4-12(5-8-15)10-14-11-13(6-9-16(14)22)20-18(24)17(23)19(25)21(27-20)28-2/h4-9,11,17-21,23-25H,3,10H2,1-2H3/t17-,18-,19+,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222245
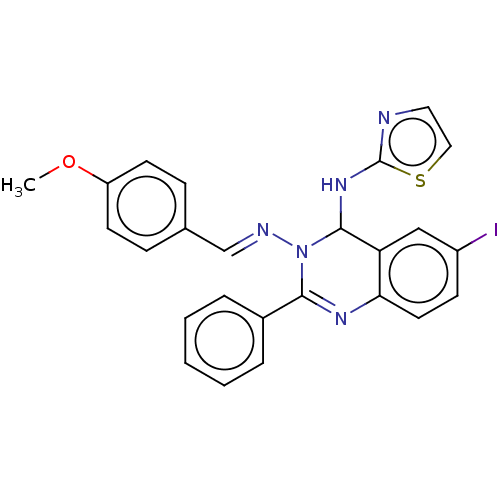
(6-Iodo-N3-(4-methoxybenzylidene)-2-phenyl-N4-(thia...)Show SMILES COc1ccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C25H20IN5OS/c1-32-20-10-7-17(8-11-20)16-28-31-23(18-5-3-2-4-6-18)29-22-12-9-19(26)15-21(22)24(31)30-25-27-13-14-33-25/h2-16,24H,1H3,(H,27,30)/b28-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222255
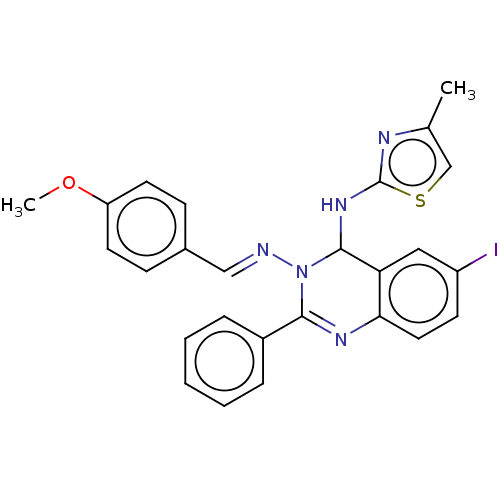
(6-Iodo-N3-(4-methoxybenzylidene)-N4-(4-methylthiaz...)Show SMILES COc1ccc(\C=N\N2C(Nc3nc(C)cs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C26H22IN5OS/c1-17-16-34-26(29-17)31-25-22-14-20(27)10-13-23(22)30-24(19-6-4-3-5-7-19)32(25)28-15-18-8-11-21(33-2)12-9-18/h3-16,25H,1-2H3,(H,29,31)/b28-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222249
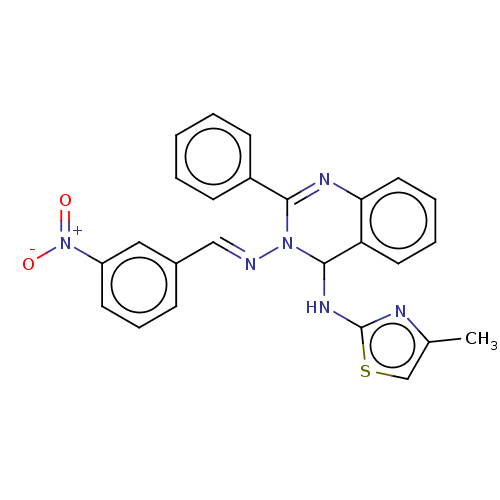
(N4-(4-Methylthiazol-2-yl)-N3-(3-nitrobenzylidene)-...)Show SMILES Cc1csc(NC2N(\N=C\c3cccc(c3)[N+]([O-])=O)C(=Nc3ccccc23)c2ccccc2)n1 |c:20| Show InChI InChI=1S/C25H20N6O2S/c1-17-16-34-25(27-17)29-24-21-12-5-6-13-22(21)28-23(19-9-3-2-4-10-19)30(24)26-15-18-8-7-11-20(14-18)31(32)33/h2-16,24H,1H3,(H,27,29)/b26-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50315426
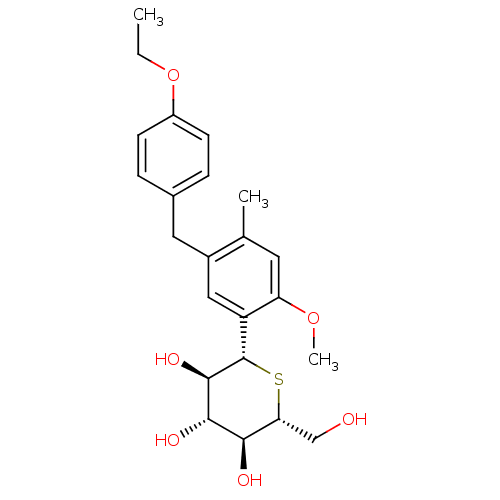
((1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4...)Show SMILES CCOc1ccc(Cc2cc([C@@H]3S[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(OC)cc2C)cc1 |r| Show InChI InChI=1S/C23H30O6S/c1-4-29-16-7-5-14(6-8-16)10-15-11-17(18(28-3)9-13(15)2)23-22(27)21(26)20(25)19(12-24)30-23/h5-9,11,19-27H,4,10,12H2,1-3H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition OF SGLT2 (unknown origin) |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50315426

((1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4...)Show SMILES CCOc1ccc(Cc2cc([C@@H]3S[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(OC)cc2C)cc1 |r| Show InChI InChI=1S/C23H30O6S/c1-4-29-16-7-5-14(6-8-16)10-15-11-17(18(28-3)9-13(15)2)23-22(27)21(26)20(25)19(12-24)30-23/h5-9,11,19-27H,4,10,12H2,1-3H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222250

(N3-(4-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...)Show SMILES COc1ccc(\C=N\N2C(Nc3nc(C)cs3)c3ccccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C26H23N5OS/c1-18-17-33-26(28-18)30-25-22-10-6-7-11-23(22)29-24(20-8-4-3-5-9-20)31(25)27-16-19-12-14-21(32-2)15-13-19/h3-17,25H,1-2H3,(H,28,30)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222240
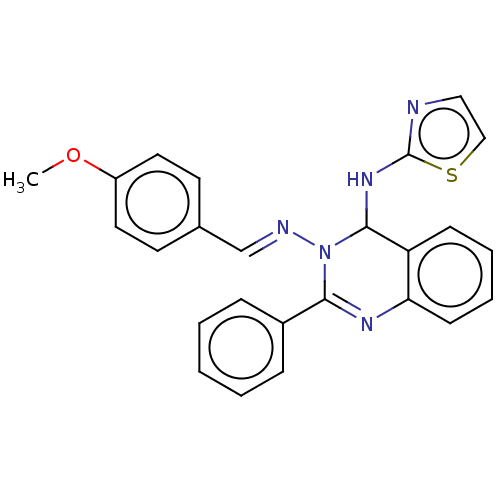
(N3-(4-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...)Show SMILES COc1ccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)cc1 |c:24| Show InChI InChI=1S/C25H21N5OS/c1-31-20-13-11-18(12-14-20)17-27-30-23(19-7-3-2-4-8-19)28-22-10-6-5-9-21(22)24(30)29-25-26-15-16-32-25/h2-17,24H,1H3,(H,26,29)/b27-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50396779
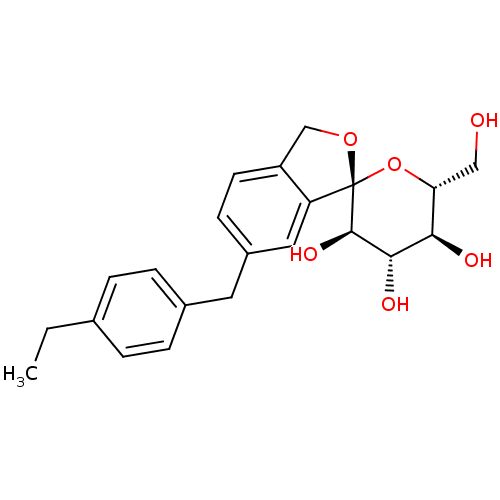
(TOFOGLIFLOZIN)Show SMILES CCc1ccc(Cc2ccc3CO[C@]4(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)c3c2)cc1 |r| Show InChI InChI=1S/C22H26O6/c1-2-13-3-5-14(6-4-13)9-15-7-8-16-12-27-22(17(16)10-15)21(26)20(25)19(24)18(11-23)28-22/h3-8,10,18-21,23-26H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50510994
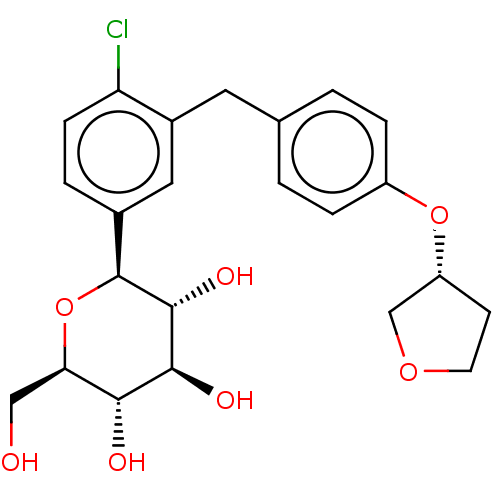
(CHEMBL4521016)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(O[C@@H]3CCOC3)cc2)c1 |r| Show InChI InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed i HEK293 cell membranes assessed as reduction in [14C]-alpha-methyl glucopyranoside uptake incubated for 4 hrs by... |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50510994

(CHEMBL4521016)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(O[C@@H]3CCOC3)cc2)c1 |r| Show InChI InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222247
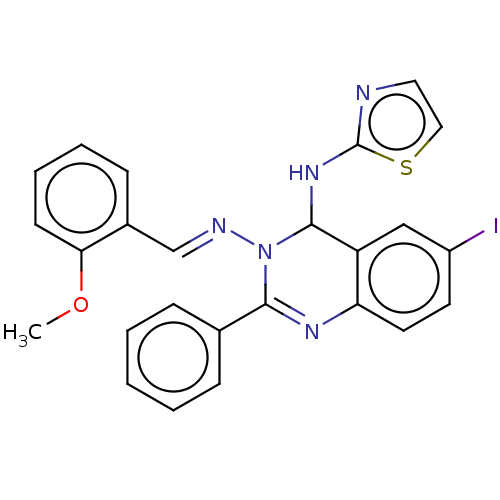
(6-Iodo-N3-(2-methoxybenzylidene)-2-phenyl-N4-(thia...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nccs2)c2cc(I)ccc2N=C1c1ccccc1 |c:28| Show InChI InChI=1S/C25H20IN5OS/c1-32-22-10-6-5-9-18(22)16-28-31-23(17-7-3-2-4-8-17)29-21-12-11-19(26)15-20(21)24(31)30-25-27-13-14-33-25/h2-16,24H,1H3,(H,27,30)/b28-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222257
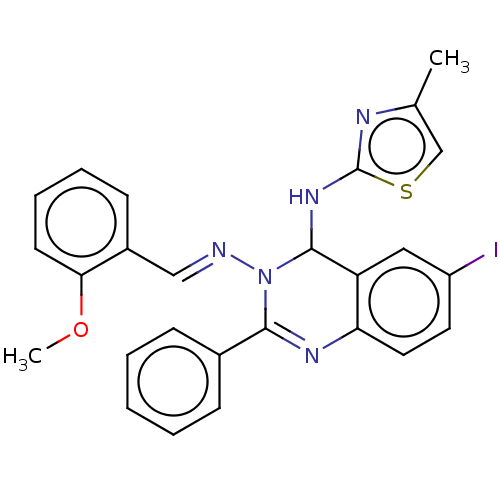
(6-Iodo-N3-(2-methoxybenzylidene)-N4-(4-methylthiaz...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nc(C)cs2)c2cc(I)ccc2N=C1c1ccccc1 |c:29| Show InChI InChI=1S/C26H22IN5OS/c1-17-16-34-26(29-17)31-25-21-14-20(27)12-13-22(21)30-24(18-8-4-3-5-9-18)32(25)28-15-19-10-6-7-11-23(19)33-2/h3-16,25H,1-2H3,(H,29,31)/b28-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222252
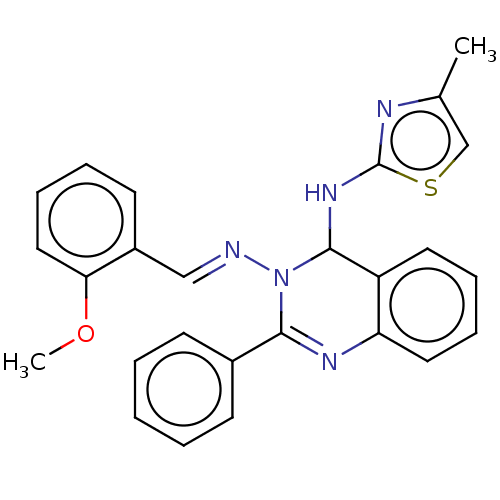
(N3-(2-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nc(C)cs2)c2ccccc2N=C1c1ccccc1 |c:28| Show InChI InChI=1S/C26H23N5OS/c1-18-17-33-26(28-18)30-25-21-13-7-8-14-22(21)29-24(19-10-4-3-5-11-19)31(25)27-16-20-12-6-9-15-23(20)32-2/h3-17,25H,1-2H3,(H,28,30)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50386885
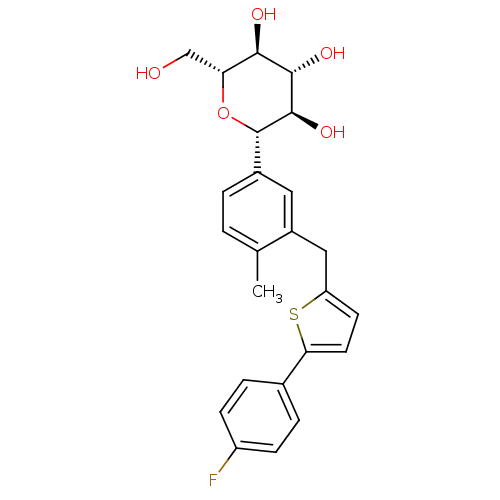
(CANAGLIFLOZIN | CANAGLIFLOZIN HYDRATE | US10752604...)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ccc(F)cc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222242
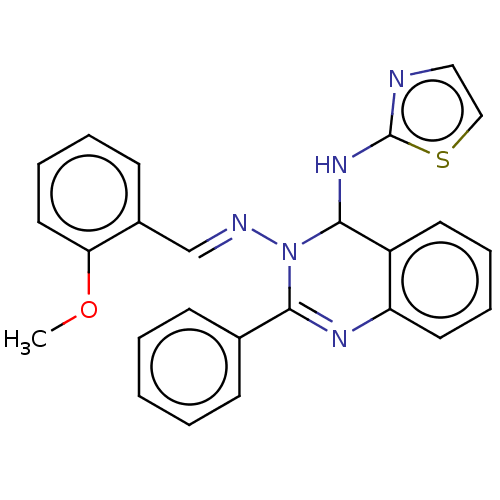
(N3-(2-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...)Show SMILES COc1ccccc1\C=N\N1C(Nc2nccs2)c2ccccc2N=C1c1ccccc1 |c:27| Show InChI InChI=1S/C25H21N5OS/c1-31-22-14-8-5-11-19(22)17-27-30-23(18-9-3-2-4-10-18)28-21-13-7-6-12-20(21)24(30)29-25-26-15-16-32-25/h2-17,24H,1H3,(H,26,29)/b27-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222256
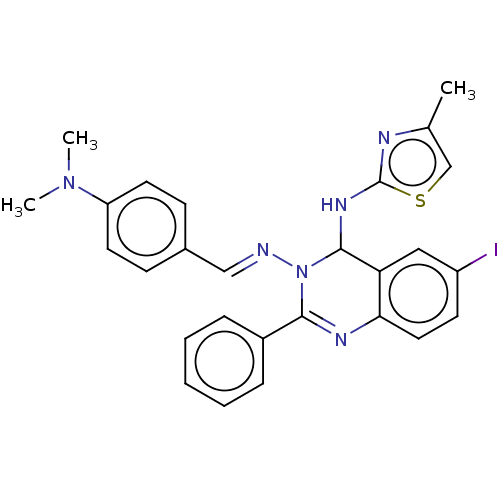
(N3-(4-(Dimethylamino)-benzylidene)-6-iodo-N4-(4-me...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nc(C)cs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:27| Show InChI InChI=1S/C27H25IN6S/c1-18-17-35-27(30-18)32-26-23-15-21(28)11-14-24(23)31-25(20-7-5-4-6-8-20)34(26)29-16-19-9-12-22(13-10-19)33(2)3/h4-17,26H,1-3H3,(H,30,32)/b29-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
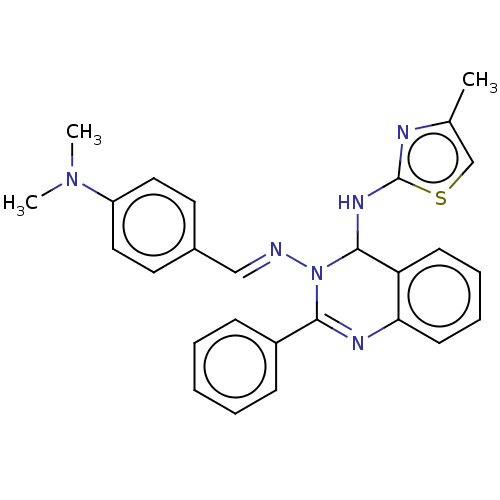
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222251
(N3-(4-(Dimethylamino)-benzylidene)-N4-(4-methylthi...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nc(C)cs3)c3ccccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C27H26N6S/c1-19-18-34-27(29-19)31-26-23-11-7-8-12-24(23)30-25(21-9-5-4-6-10-21)33(26)28-17-20-13-15-22(16-14-20)32(2)3/h4-18,26H,1-3H3,(H,29,31)/b28-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222253

(N3-Benzylidene-6-iodo-N4-(4-methylthiazol-2-yl)-2-...)Show SMILES Cc1csc(NC2N(\N=C\c3ccccc3)C(=Nc3ccc(I)cc23)c2ccccc2)n1 |c:17| Show InChI InChI=1S/C25H20IN5S/c1-17-16-32-25(28-17)30-24-21-14-20(26)12-13-22(21)29-23(19-10-6-3-7-11-19)31(24)27-15-18-8-4-2-5-9-18/h2-16,24H,1H3,(H,28,30)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381554
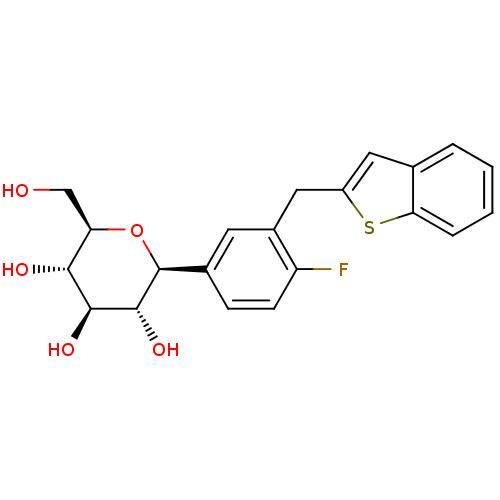
(CHEMBL2018096)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(F)c(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H21FO5S/c22-15-6-5-12(21-20(26)19(25)18(24)16(10-23)27-21)7-13(15)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-21,23-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222246
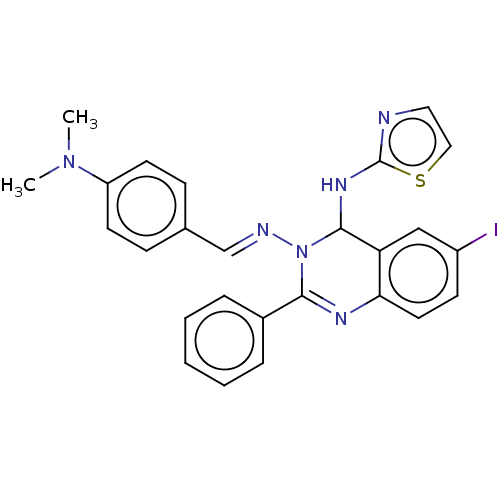
(N3-{4-(Dimethylamino)-benzylidene}-6-iodo-2-phenyl...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:26| Show InChI InChI=1S/C26H23IN6S/c1-32(2)21-11-8-18(9-12-21)17-29-33-24(19-6-4-3-5-7-19)30-23-13-10-20(27)16-22(23)25(33)31-26-28-14-15-34-26/h3-17,25H,1-2H3,(H,28,31)/b29-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50466848
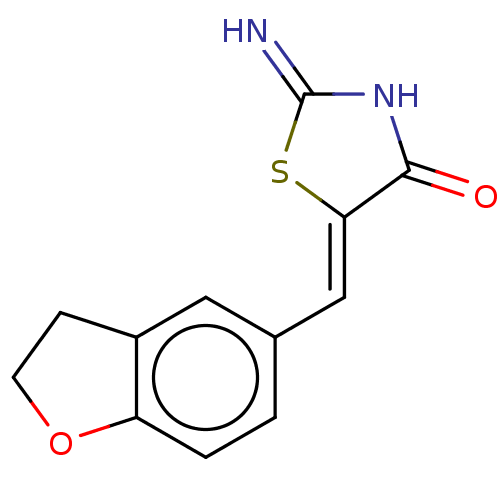
(CHEMBL4283751)Show InChI InChI=1S/C12H10N2O2S/c13-12-14-11(15)10(17-12)6-7-1-2-9-8(5-7)3-4-16-9/h1-2,5-6H,3-4H2,(H2,13,14,15)/b10-6- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222248
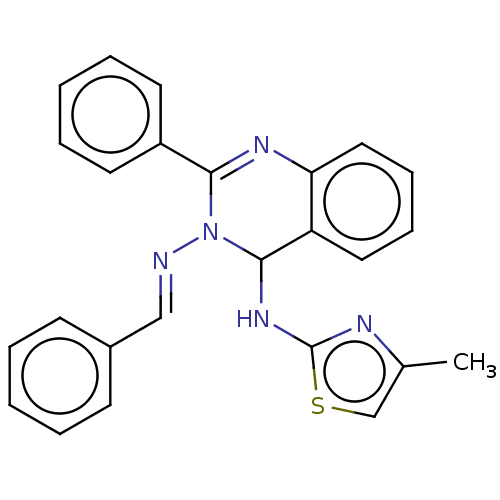
(N3-Benzylidene-N4-(4-methylthiazol-2-yl)-2-phenylq...)Show SMILES Cc1csc(NC2N(\N=C\c3ccccc3)C(=Nc3ccccc23)c2ccccc2)n1 |c:17| Show InChI InChI=1S/C25H21N5S/c1-18-17-31-25(27-18)29-24-21-14-8-9-15-22(21)28-23(20-12-6-3-7-13-20)30(24)26-16-19-10-4-2-5-11-19/h2-17,24H,1H3,(H,27,29)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222241
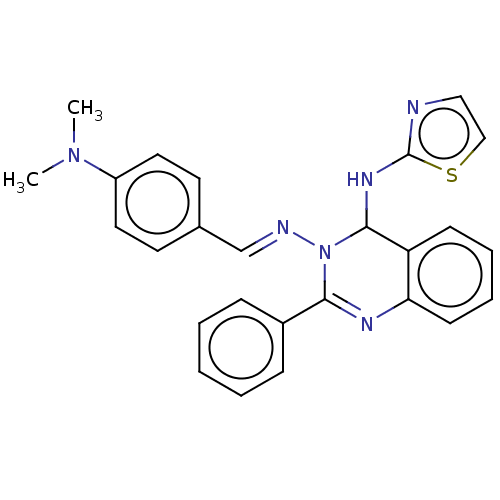
(N3-(4-(Dimethylamino)-benzylidene)-2-phenyl-N4-(th...)Show SMILES CN(C)c1ccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C26H24N6S/c1-31(2)21-14-12-19(13-15-21)18-28-32-24(20-8-4-3-5-9-20)29-23-11-7-6-10-22(23)25(32)30-26-27-16-17-33-26/h3-18,25H,1-2H3,(H,27,30)/b28-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222243
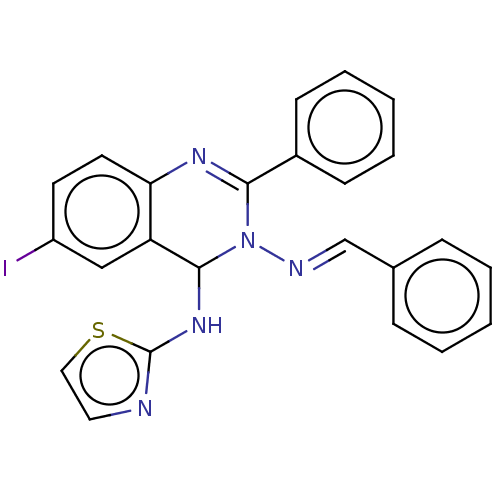
(N3-Benzylidene-6-iodo-2-phenyl-N4-(thiazol-2-yl)-q...)Show SMILES Ic1ccc2N=C(N(\N=C\c3ccccc3)C(Nc3nccs3)c2c1)c1ccccc1 |c:5| Show InChI InChI=1S/C24H18IN5S/c25-19-11-12-21-20(15-19)23(29-24-26-13-14-31-24)30(27-16-17-7-3-1-4-8-17)22(28-21)18-9-5-2-6-10-18/h1-16,23H,(H,26,29)/b27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222238
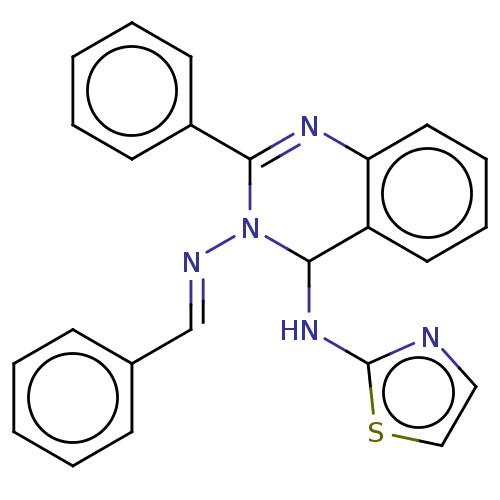
(N3-Benzylidene-2-phenyl-N4-(thiazol-2-yl)-quinazol...)Show SMILES N(C1N(\N=C\c2ccccc2)C(=Nc2ccccc12)c1ccccc1)c1nccs1 |c:12| Show InChI InChI=1S/C24H19N5S/c1-3-9-18(10-4-1)17-26-29-22(19-11-5-2-6-12-19)27-21-14-8-7-13-20(21)23(29)28-24-25-15-16-30-24/h1-17,23H,(H,25,28)/b26-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
| Assay Description
The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... |
Bioorg Chem 71: 181-191 (2017)
Article DOI: 10.1016/j.bioorg.2017.02.004
BindingDB Entry DOI: 10.7270/Q2PR7TTR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081174
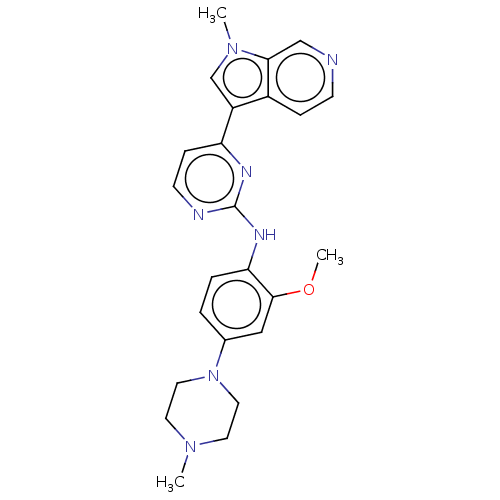
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Presenilin-1
(Homo sapiens (Human)) | BDBM50613787
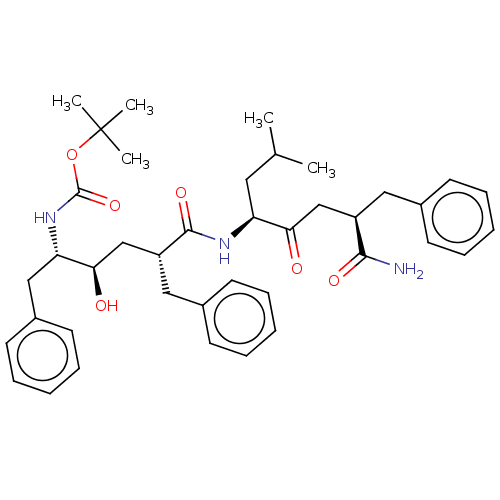
(CHEMBL5283881)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)C[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50081174

(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50162287
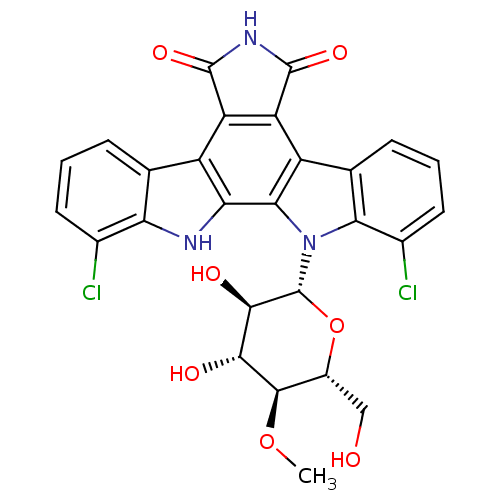
((Rebeccamycin)1,11-dichloro-12-(3,4-dihydroxy-6-hy...)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H]([C@H](O)[C@H]1O)n1c2c(Cl)cccc2c2c3C(=O)NC(=O)c3c3c4cccc(Cl)c4[nH]c3c12 |r| Show InChI InChI=1S/C27H21Cl2N3O7/c1-38-24-13(8-33)39-27(23(35)22(24)34)32-20-10(5-3-7-12(20)29)15-17-16(25(36)31-26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)32/h2-7,13,22-24,27,30,33-35H,8H2,1H3,(H,31,36,37)/t13-,22-,23-,24-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM100152
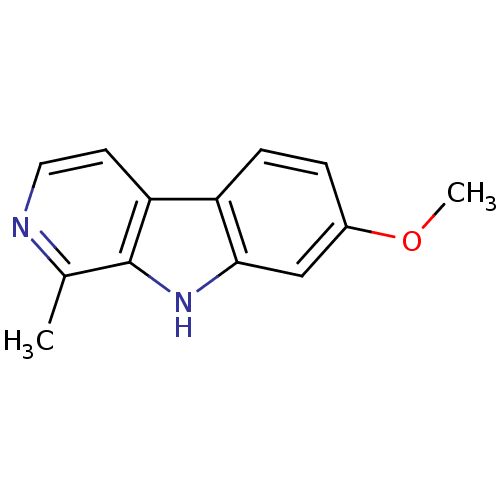
(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50235017

(LP-802034 | LX-4211 | Sotagliflozin)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5S/c1-3-26-15-7-4-12(5-8-15)10-14-11-13(6-9-16(14)22)20-18(24)17(23)19(25)21(27-20)28-2/h4-9,11,17-21,23-25H,3,10H2,1-2H3/t17-,18-,19+,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20875
(1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...)Show SMILES OC[C@H]1O[C@@H](Oc2cc(O)cc(O)c2C(=O)CCc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C21H24O10/c22-9-16-18(27)19(28)20(29)21(31-16)30-15-8-12(24)7-14(26)17(15)13(25)6-3-10-1-4-11(23)5-2-10/h1-2,4-5,7-8,16,18-24,26-29H,3,6,9H2/t16-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111773
BindingDB Entry DOI: 10.7270/Q2TT4V97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50347750
(CHEMBL1802358)Show SMILES O=C1NC(Nc2ccccc2)=NC1=Cc1ccc2OCOc2c1 |w:13.15,c:11| Show InChI InChI=1S/C17H13N3O3/c21-16-13(8-11-6-7-14-15(9-11)23-10-22-14)19-17(20-16)18-12-4-2-1-3-5-12/h1-9H,10H2,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50613787

(CHEMBL5283881)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)C[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50613785
(CHEMBL5288413)Show SMILES NCCC(NC(=O)c1ccc(Cl)c(NC(=O)c2cc3cnc(CC(N)=O)nc3[nH]c2=O)c1)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50372905

(CHEMBL270636)Show SMILES CCOc1cc(C=C2SC(S)=NC2=O)ccc1OC |w:6.5,c:10| Show InChI InChI=1S/C13H13NO3S2/c1-3-17-10-6-8(4-5-9(10)16-2)7-11-12(15)14-13(18)19-11/h4-7H,3H2,1-2H3,(H,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50613787

(CHEMBL5283881)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)C[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50613778

(CHEMBL513265) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data