

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123752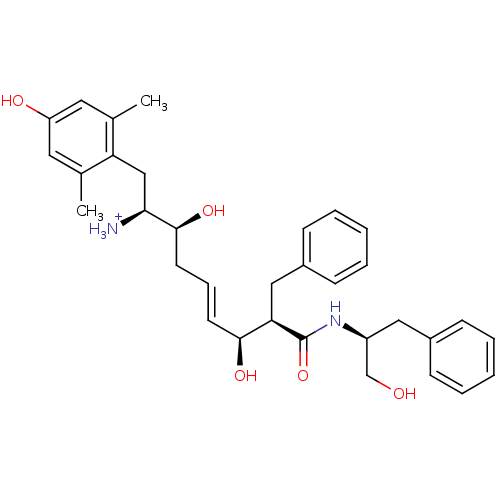 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123763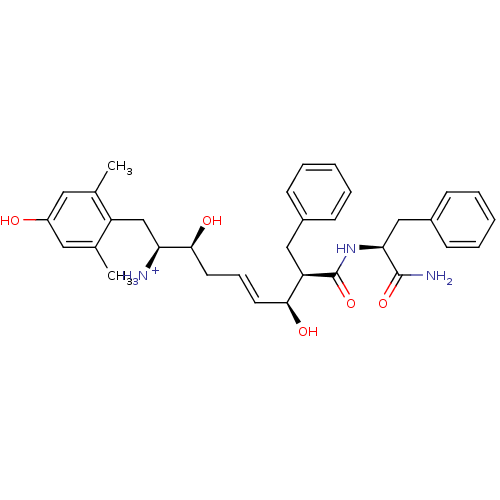 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123754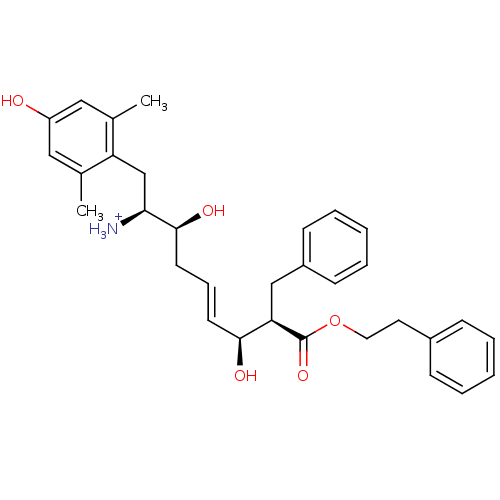 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123756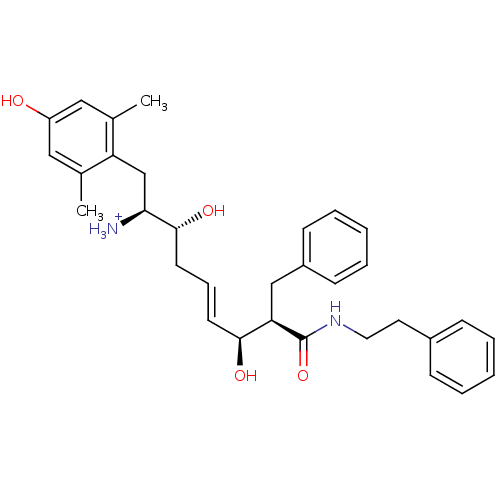 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123750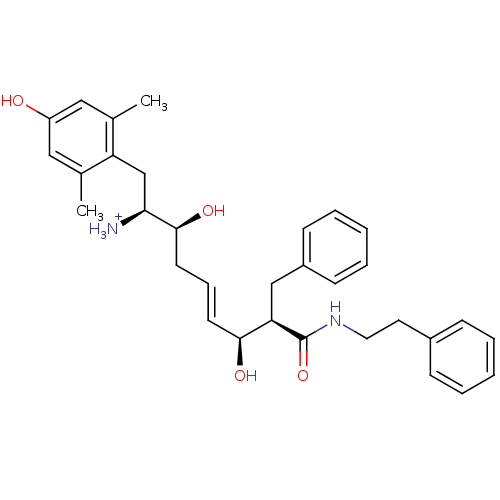 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123751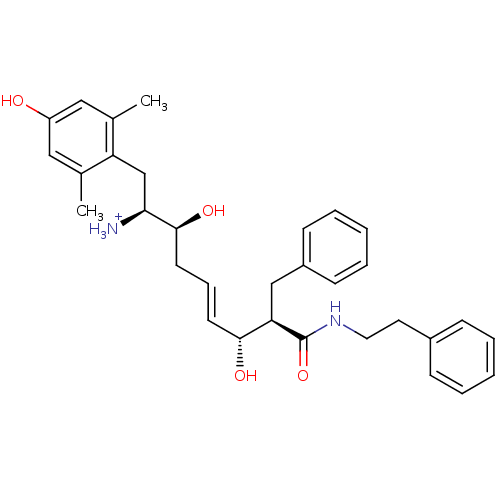 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123759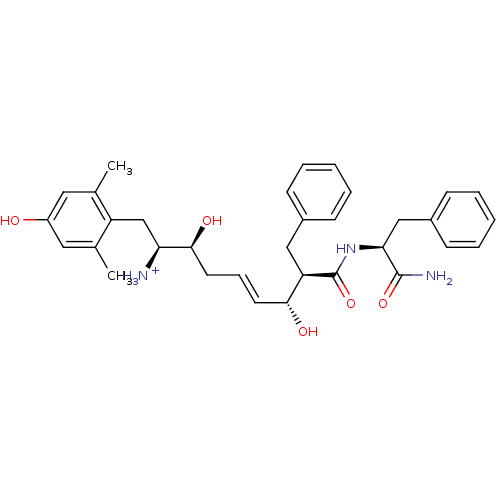 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123762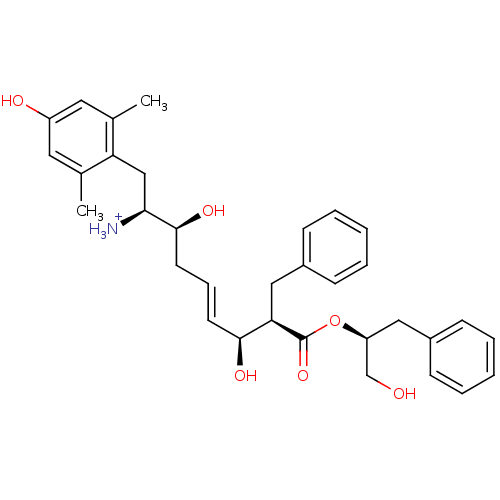 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123760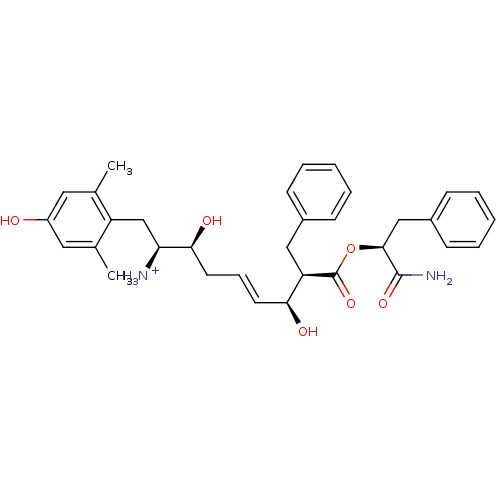 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123757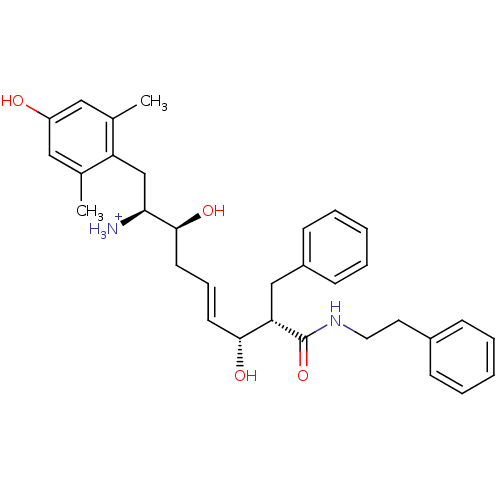 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123755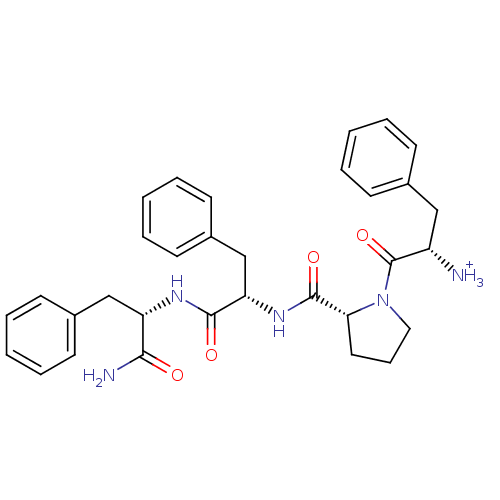 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123761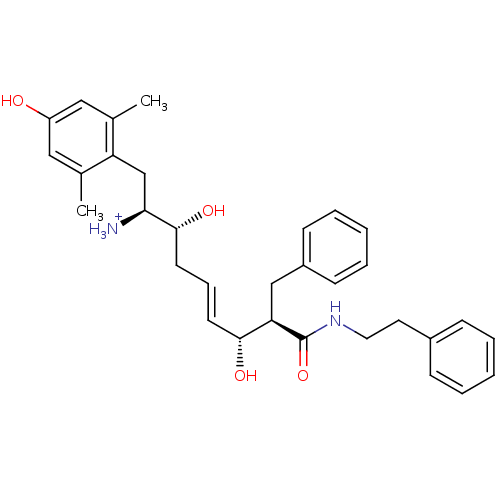 ((E)-(1S,2R,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123753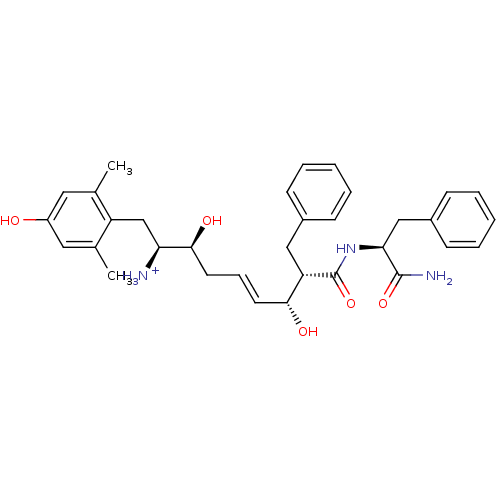 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123758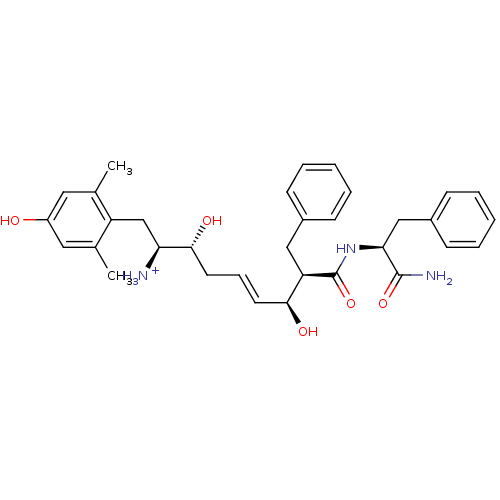 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123749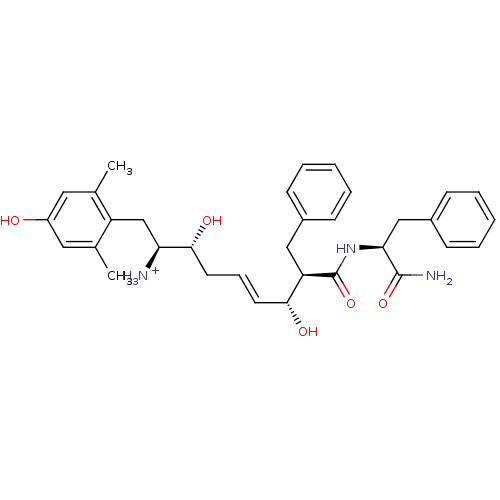 ((E)-(1S,2R,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123758 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123762 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410097 (US10370370, Compound 2-P1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123760 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123759 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123763 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123749 ((E)-(1S,2R,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410113 (US10370370, Compound 9-P2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123751 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410139 (US10370370, Compound (R)-31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322432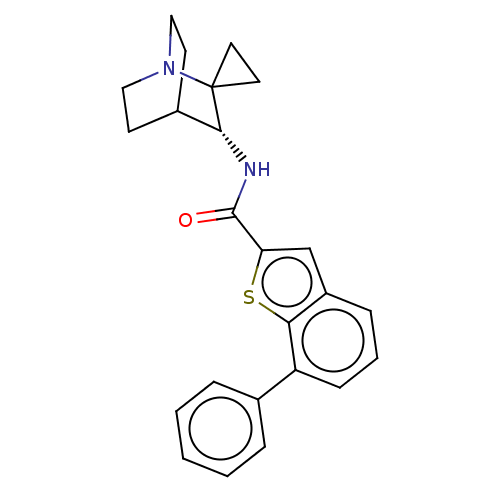 ((R)-7-phenyl-N-(1'-azaspiro[cyclopropane-1,2'-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410099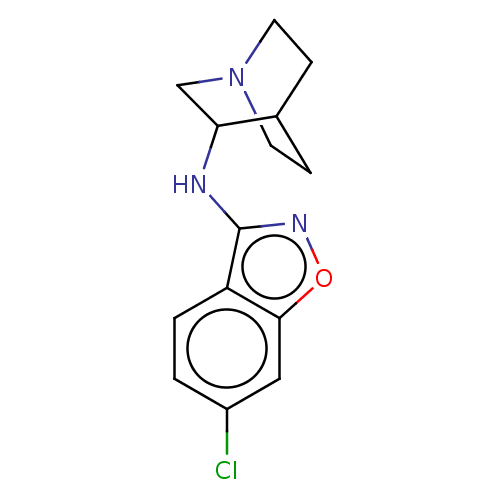 (US10370370, Compound 3-P1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410109 (US10370370, Compound 7-P2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123759 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322406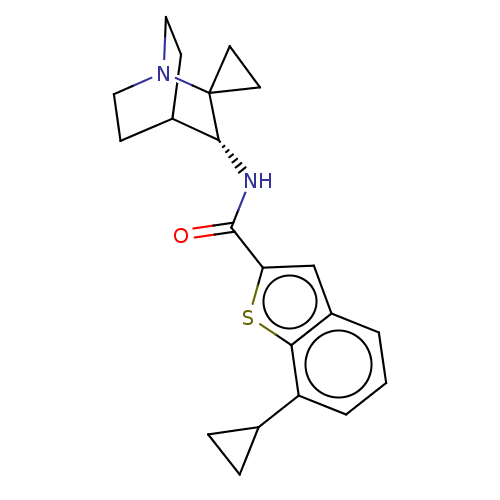 ((R)-7-cyclopropyl-N-(1'-azaspiro[cyclopropane-1,2'...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322274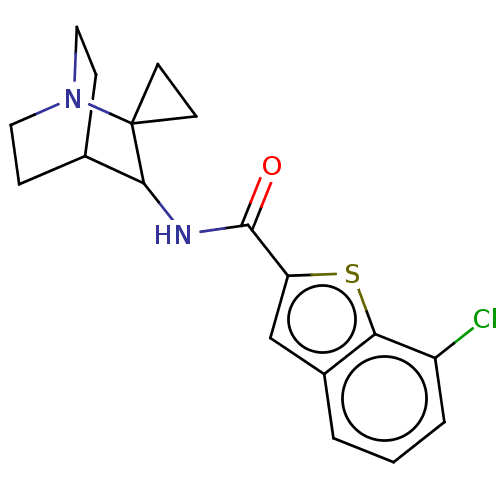 (7-chloro-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123763 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123750 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410112 (US10370370, Compound 9-P1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 43.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410152 (US10370370, Compound (R)-43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410148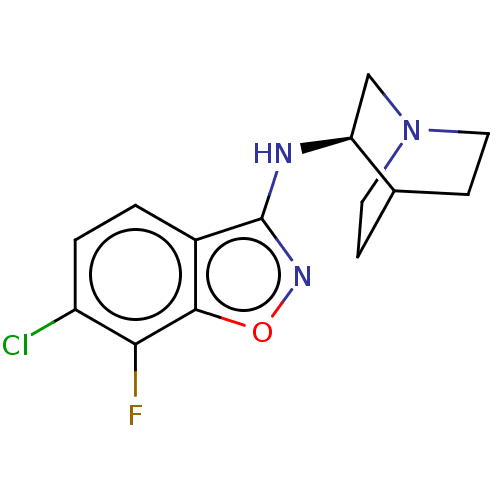 (US10370370, Compound (R)-41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123758 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322452 ((R)-6-chloro-7-methyl-N-(1'-azaspiro[cyclopropane-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM322274 (7-chloro-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., ... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123753 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123756 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123752 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322387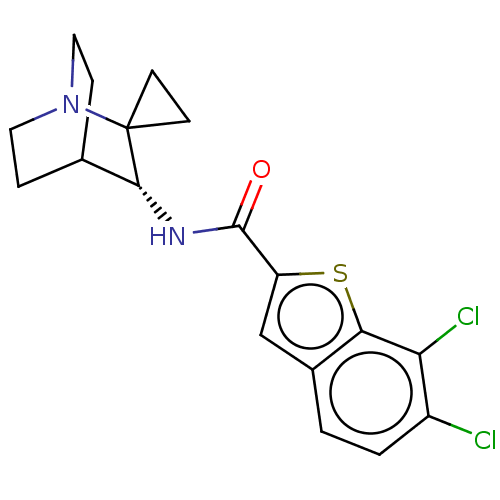 ((R)-6,7-dichloro-N-(1'-azaspiro[cyclopropane-1,2'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 54.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123754 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM410101 (US10370370, Compound (R)-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description h-5HT3: In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Followi... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322402 ((R)-7-methoxy-N-(1'-azaspiro[cyclopropane-1,2'-bic...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM410112 (US10370370, Compound 9-P1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 59.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description α7 nAChR: The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affin... | US Patent US10370370 (2019) BindingDB Entry DOI: 10.7270/Q2348NRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123757 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM322271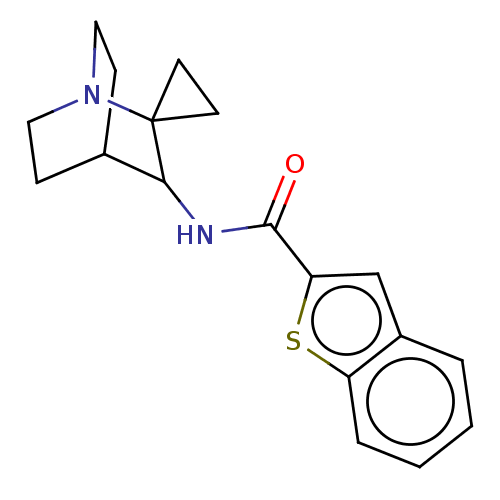 (N-(1''-azaspiro[cyclopropane-1,2''-bicyclo[2.2.2]o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axovant Sciences GmbH US Patent | Assay Description The ability of compounds to displace binding of radioactive ligands from human α7 nAChR was determined, as a measure of the affinity of the comp... | US Patent US10183938 (2019) BindingDB Entry DOI: 10.7270/Q2VD71JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123751 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1374 total ) | Next | Last >> |