

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50104398 ((R)-2-Methyl-6-(4-nitro-phenylsulfanyl)-tetrahydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-1 (Homo sapiens (Human)) | BDBM50355656 (CHEMBL1910970) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50355665 (CHEMBL1910971) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50355656 (CHEMBL1910970) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-3 (Homo sapiens (Human)) | BDBM50355657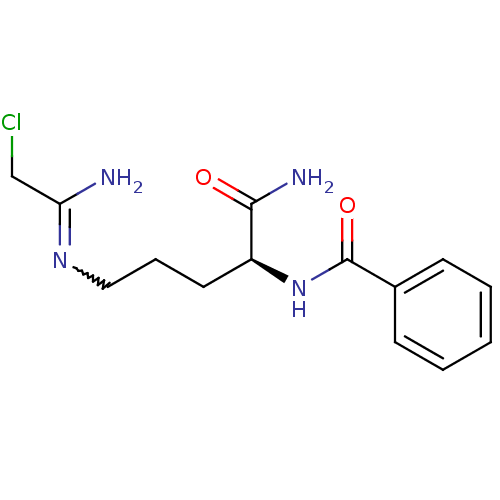 (CHEMBL1910972 | CHEMBL1962361) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-3 (Homo sapiens (Human)) | BDBM50355665 (CHEMBL1910971) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287679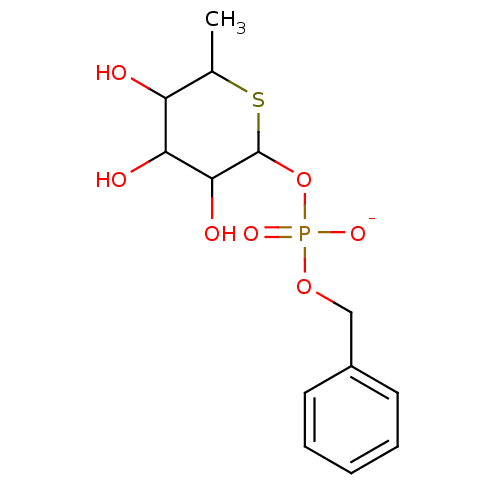 (CHEMBL61555 | Sodium; benzyl 3,4,5-trihydroxy-6-me...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50104411 ((R)-6-Methyl-tetrahydro-thiopyran-2,3,4,5-tetraol ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-1 (Homo sapiens (Human)) | BDBM50355657 (CHEMBL1910972 | CHEMBL1962361) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-1 (Homo sapiens (Human)) | BDBM50355658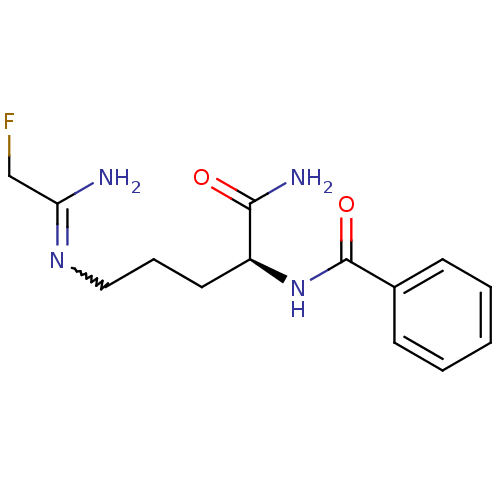 (CHEMBL1910973) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287680 ((3R,4S,5S)-2-Methyl-6-(4-nitro-phenoxy)-tetrahydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50355657 (CHEMBL1910972 | CHEMBL1962361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287682 ((3S,4S,5R)-2-Cyclohexyloxy-6-methyl-tetrahydro-thi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-3 (Homo sapiens (Human)) | BDBM50355658 (CHEMBL1910973) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD3 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50355658 (CHEMBL1910973) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Irreversible inhibition of PAD4 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... | J Med Chem 54: 6919-35 (2011) Article DOI: 10.1021/jm2008985 BindingDB Entry DOI: 10.7270/Q24F1R4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287681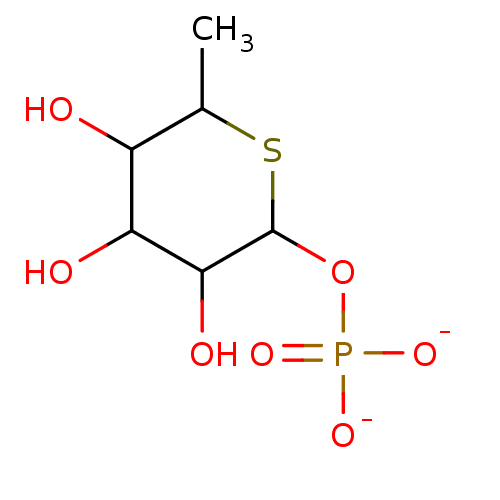 (CHEMBL59958 | Sodium; Phosphoric acid mono-((3S,4S...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287683 ((3S,4S,5R)-2-Methoxy-6-methyl-tetrahydro-thiopyran...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50287678 ((3R,4S,5S)-2-Methyl-6-methylsulfanyl-tetrahydro-th...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against alpha-L-fucosidase from bovine epididymis | Bioorg Med Chem Lett 6: 1989-1992 (1996) Article DOI: 10.1016/0960-894X(96)00356-3 BindingDB Entry DOI: 10.7270/Q2PR7W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK2 (880 to end residues) expressed in baculovirus infected Sf21 cells using TK-substrate-biotin as substra... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545650 (Delgocitinib | JTE-052 | JTE-052A | LEO 124249 | L...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK2 (880 to end residues) expressed in baculovirus infected Sf21 cells using TK-substrate-biotin as substra... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50545650 (Delgocitinib | JTE-052 | JTE-052A | LEO 124249 | L...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK1 (850 to 1154 residues) expressed in baculovirus expression system using TK-substrate-biotin as substrat... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK1 (850 to 1154 residues) expressed in baculovirus expression system using TK-substrate-biotin as substrat... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532190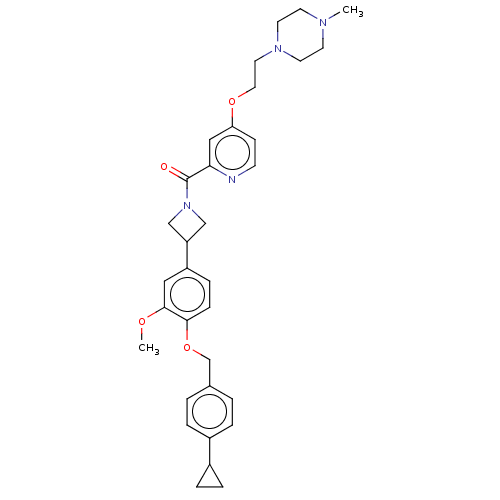 (CHEMBL4452717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109867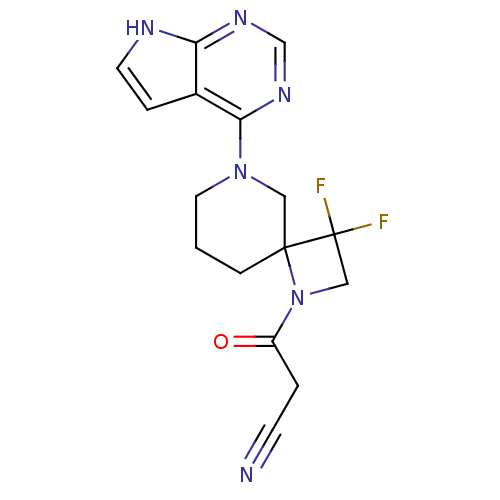 (US8609647, 27 | US8609647, 37 | US8609647, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109867 (US8609647, 27 | US8609647, 37 | US8609647, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109863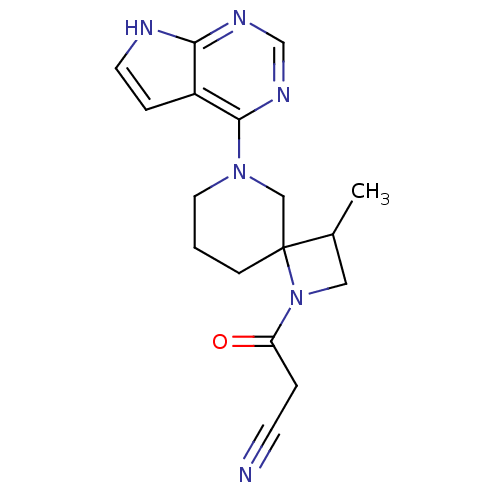 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109863 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532193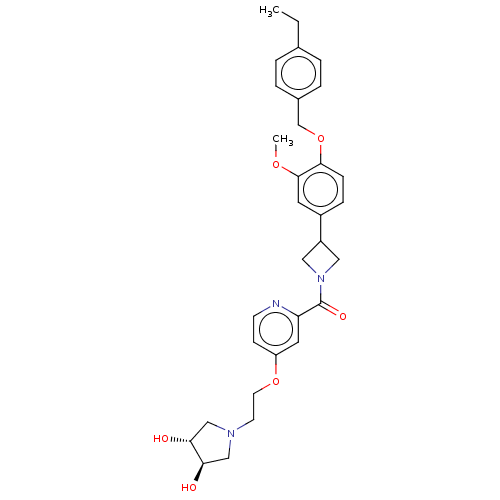 (CHEMBL4539279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50370763 (CHEMBL1203926) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA polymerase | J Med Chem 49: 6950-3 (2006) Article DOI: 10.1021/jm0610245 BindingDB Entry DOI: 10.7270/Q2XD12GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50503530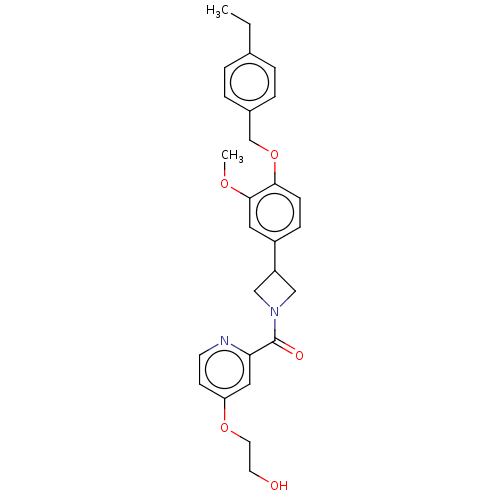 (CHEMBL4437645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 115-118 (2019) Article DOI: 10.1016/j.bmcl.2018.10.051 BindingDB Entry DOI: 10.7270/Q2RN3C4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50503530 (CHEMBL4437645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109907 (US8609647, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532178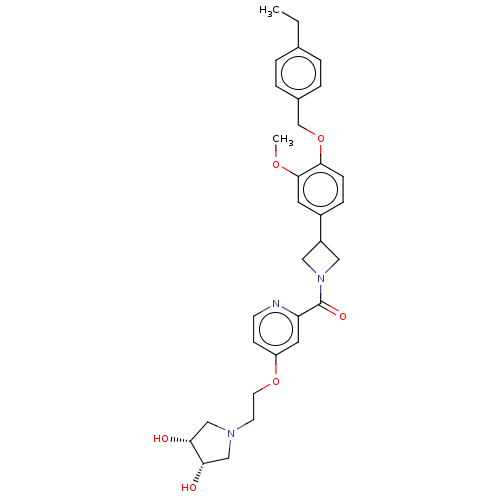 (CHEMBL4460662) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109866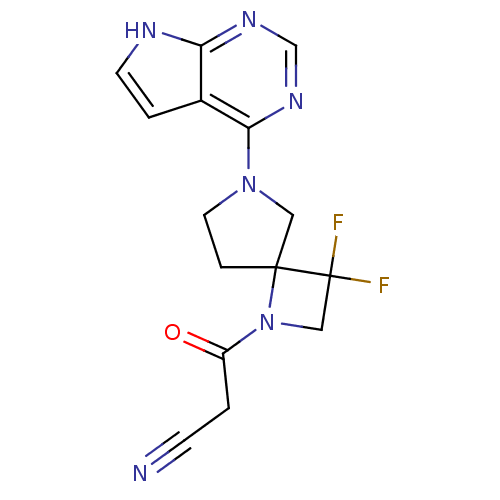 (US8609647, 26 | US8609647, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532197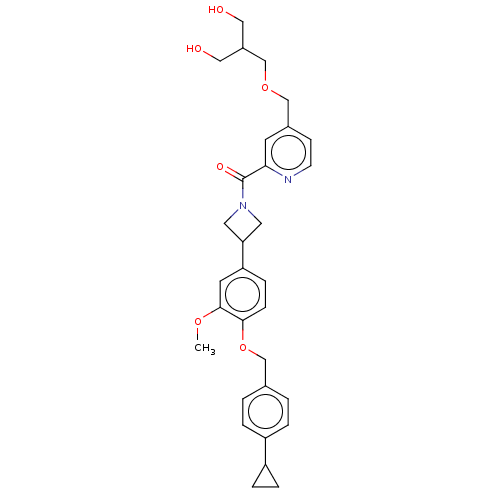 (CHEMBL4522278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109866 (US8609647, 26 | US8609647, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50191541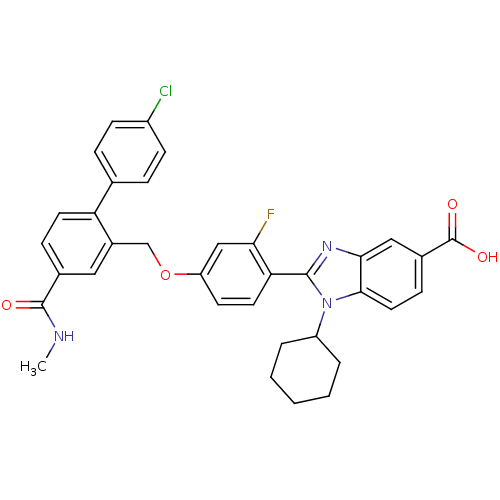 (2-[4-(4'-chloro-4-methylcarbamoylbiphenyl-2-ylmeth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50191533 (2-[4-(4'-chloro-4-dimethylcarbamoylbiphenyl-2-ylme...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532180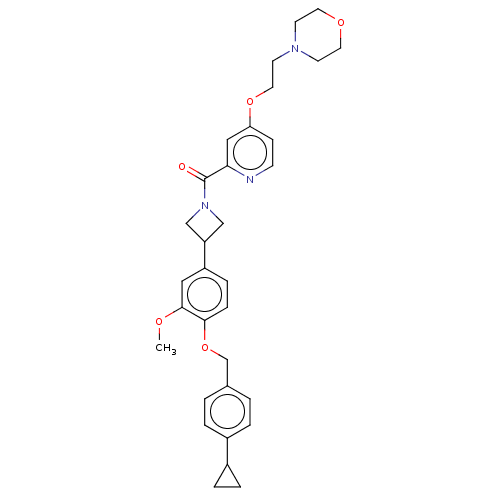 (CHEMBL4473704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532183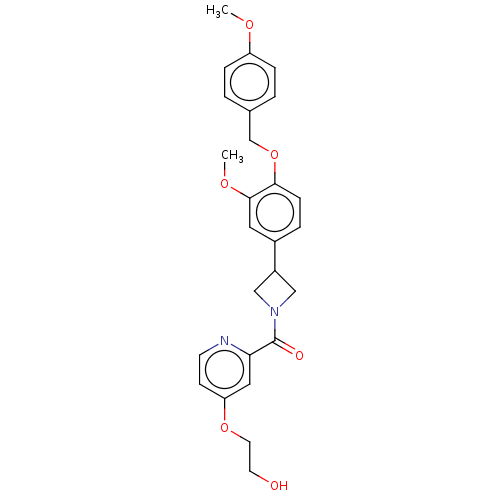 (CHEMBL4543929) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532194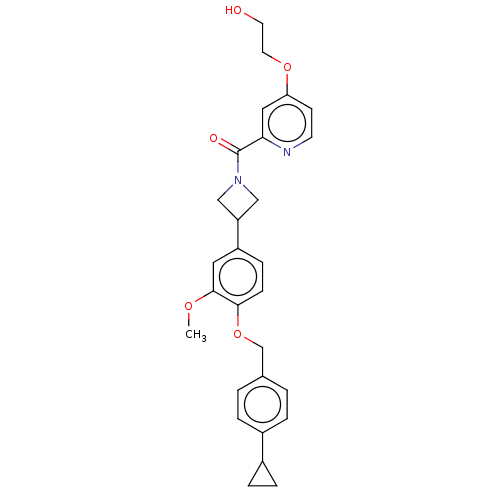 (CHEMBL4575001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110994 (4-[2-Methyl-4-(5-methyl-thiophen-2-yl)-oxazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human purified Prostaglandin G/H synthase 2 | J Med Chem 45: 1511-7 (2002) BindingDB Entry DOI: 10.7270/Q2H995XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50191549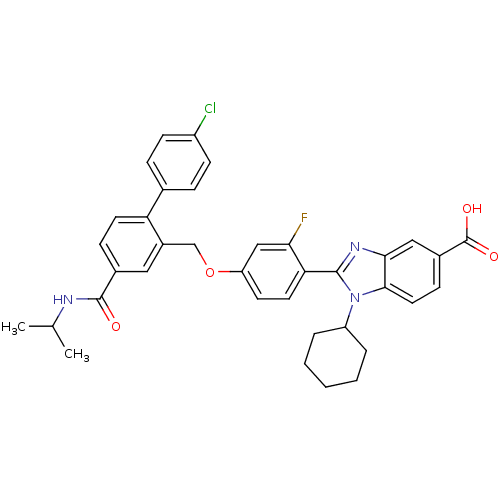 (2-[4-(4'-chloro-4-isopropylcarbamoylbiphenyl-2-ylm...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532188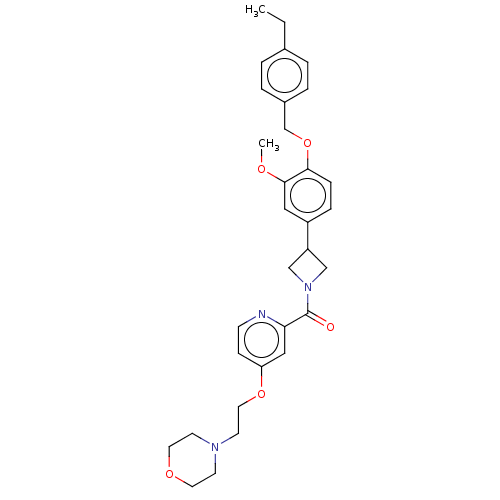 (CHEMBL4570496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50532195 (CHEMBL4555993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human CSF1R tyrosine kinase domain (538 to 972 residues) using Poly(Glu, Tyr)-biotinylated peptide as substrate preincubated for 5 mins... | Bioorg Med Chem Lett 29: 873-877 (2019) Article DOI: 10.1016/j.bmcl.2019.02.006 BindingDB Entry DOI: 10.7270/Q22F7RXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50545650 (Delgocitinib | JTE-052 | JTE-052A | LEO 124249 | L...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50191505 (2-[4-(4-carbamoyl-4'-chlorobiphenyl-2-ylmethoxy)ph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50158838 (2-{4-[2-(4-chlorophenyl)-5-(4-hydroxyhexahydro-1-p...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50191508 (2-{4-[4'-chloro-4-(morpholine-4-carbonyl)biphenyl-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HCV 1b BK six His-tagged C-terminal truncated 544 amino acid NS5B RNA dependent RNA polymerase | J Med Chem 49: 4721-36 (2006) Article DOI: 10.1021/jm060269e BindingDB Entry DOI: 10.7270/Q2FN15TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 710 total ) | Next | Last >> |