 Found 693 hits with Last Name = 'he' and Initial = 'jx'
Found 693 hits with Last Name = 'he' and Initial = 'jx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50080924
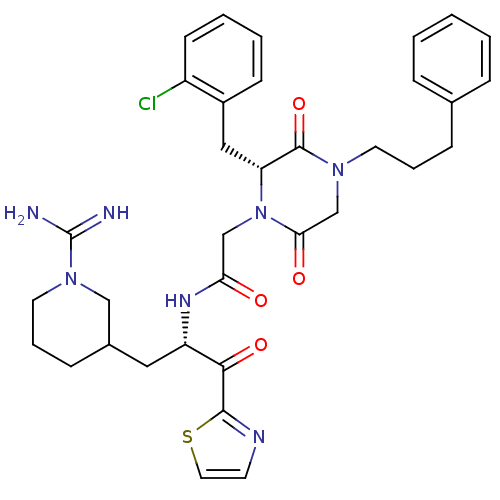
(CHEMBL83260 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3Cl)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H40ClN7O4S/c35-26-13-5-4-12-25(26)19-28-33(46)40(15-6-10-23-8-2-1-3-9-23)22-30(44)42(28)21-29(43)39-27(31(45)32-38-14-17-47-32)18-24-11-7-16-41(20-24)34(36)37/h1-5,8-9,12-14,17,24,27-28H,6-7,10-11,15-16,18-22H2,(H3,36,37)(H,39,43)/t24?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289562

(CHEMBL3216390 | CHEMBL543774 | {(R)-1-Cyclohexylme...)Show SMILES Cl.Cl.NC(=N)NCCCNC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O |r| Show InChI InChI=1S/C21H38N6O4/c22-21(23)25-11-6-10-24-19(30)17-9-4-5-12-27(17)20(31)16(26-14-18(28)29)13-15-7-2-1-3-8-15/h15-17,26H,1-14H2,(H,24,30)(H,28,29)(H4,22,23,25)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Selective inhibition of thrombin in several in vitro and in vivo models of thrombosis |
Bioorg Med Chem Lett 7: 1563-1566 (1997)
Article DOI: 10.1016/S0960-894X(97)00271-0
BindingDB Entry DOI: 10.7270/Q27W6C68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50080911

(2-((S)-4-Benzyl-2-naphthalen-2-ylmethyl-3,6-dioxo-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](Cc3ccc4ccccc4c3)C(=O)N(Cc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C36H39N7O4S/c37-36(38)41-15-6-9-26(21-41)18-29(33(46)34-39-14-16-48-34)40-31(44)22-43-30(19-25-12-13-27-10-4-5-11-28(27)17-25)35(47)42(23-32(43)45)20-24-7-2-1-3-8-24/h1-5,7-8,10-14,16-17,26,29-30H,6,9,15,18-23H2,(H3,37,38)(H,40,44)/t26?,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against thrombin was determined |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080920

(CHEMBL313769 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccc(Cl)c(Cl)c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H39Cl2N7O4S/c35-25-11-10-23(16-26(25)36)18-28-33(47)41(13-4-8-22-6-2-1-3-7-22)21-30(45)43(28)20-29(44)40-27(31(46)32-39-12-15-48-32)17-24-9-5-14-42(19-24)34(37)38/h1-3,6-7,10-12,15-16,24,27-28H,4-5,8-9,13-14,17-21H2,(H3,37,38)(H,40,44)/t24?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50080895
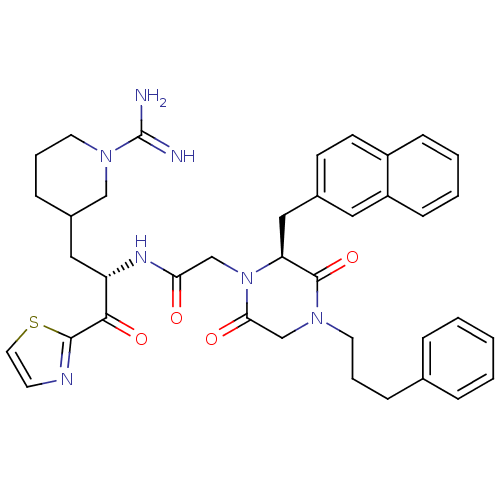
(CHEMBL311198 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](Cc3ccc4ccccc4c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C38H43N7O4S/c39-38(40)44-18-7-11-28(23-44)21-31(35(48)36-41-16-19-50-36)42-33(46)24-45-32(22-27-14-15-29-12-4-5-13-30(29)20-27)37(49)43(25-34(45)47)17-6-10-26-8-2-1-3-9-26/h1-5,8-9,12-16,19-20,28,31-32H,6-7,10-11,17-18,21-25H2,(H3,39,40)(H,42,46)/t28?,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289562

(CHEMBL3216390 | CHEMBL543774 | {(R)-1-Cyclohexylme...)Show SMILES Cl.Cl.NC(=N)NCCCNC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O |r| Show InChI InChI=1S/C21H38N6O4/c22-21(23)25-11-6-10-24-19(30)17-9-4-5-12-27(17)20(31)16(26-14-18(28)29)13-15-7-2-1-3-8-15/h15-17,26H,1-14H2,(H,24,30)(H,28,29)(H4,22,23,25)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin was evaulated |
Bioorg Med Chem Lett 7: 1563-1566 (1997)
Article DOI: 10.1016/S0960-894X(97)00271-0
BindingDB Entry DOI: 10.7270/Q27W6C68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50080916

(CHEMBL84084 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3cccc(Cl)c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H40ClN7O4S/c35-26-12-4-9-24(17-26)19-28-33(46)40(14-5-10-23-7-2-1-3-8-23)22-30(44)42(28)21-29(43)39-27(31(45)32-38-13-16-47-32)18-25-11-6-15-41(20-25)34(36)37/h1-4,7-9,12-13,16-17,25,27-28H,5-6,10-11,14-15,18-22H2,(H3,36,37)(H,39,43)/t25?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080908

(2-[(S)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...)Show SMILES [H][C@@]1(C[C@H](NC(=O)CN2[C@@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)CCCN(C1)C(N)=N Show InChI InChI=1S/C34H41N7O4S/c35-34(36)40-17-8-14-26(21-40)19-27(31(44)32-37-15-18-46-32)38-29(42)22-41-28(20-25-11-5-2-6-12-25)33(45)39(23-30(41)43)16-7-13-24-9-3-1-4-10-24/h1-6,9-12,15,18,26-28H,7-8,13-14,16-17,19-23H2,(H3,35,36)(H,38,42)/t26?,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080895

(CHEMBL311198 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](Cc3ccc4ccccc4c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C38H43N7O4S/c39-38(40)44-18-7-11-28(23-44)21-31(35(48)36-41-16-19-50-36)42-33(46)24-45-32(22-27-14-15-29-12-4-5-13-30(29)20-27)37(49)43(25-34(45)47)17-6-10-26-8-2-1-3-9-26/h1-5,8-9,12-16,19-20,28,31-32H,6-7,10-11,17-18,21-25H2,(H3,39,40)(H,42,46)/t28?,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080915
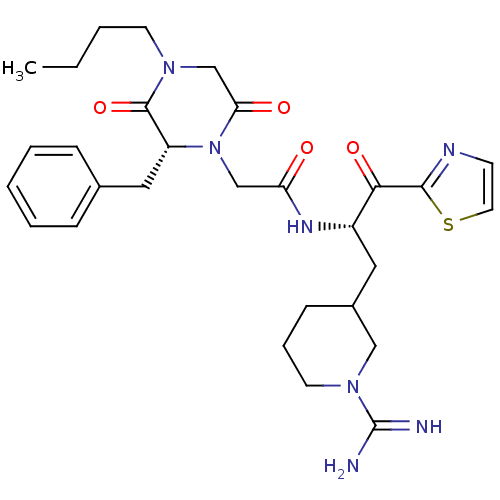
(2-((R)-2-Benzyl-4-butyl-3,6-dioxo-piperazin-1-yl)-...)Show SMILES CCCCN1CC(=O)N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)[C@H](Cc2ccccc2)C1=O Show InChI InChI=1S/C29H39N7O4S/c1-2-3-12-34-19-25(38)36(23(28(34)40)16-20-8-5-4-6-9-20)18-24(37)33-22(26(39)27-32-11-14-41-27)15-21-10-7-13-35(17-21)29(30)31/h4-6,8-9,11,14,21-23H,2-3,7,10,12-13,15-19H2,1H3,(H3,30,31)(H,33,37)/t21?,22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080903

(CHEMBL83549 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](Cc3ccc(Cl)cc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H40ClN7O4S/c35-26-12-10-24(11-13-26)19-28-33(46)40(15-4-8-23-6-2-1-3-7-23)22-30(44)42(28)21-29(43)39-27(31(45)32-38-14-17-47-32)18-25-9-5-16-41(20-25)34(36)37/h1-3,6-7,10-14,17,25,27-28H,4-5,8-9,15-16,18-22H2,(H3,36,37)(H,39,43)/t25?,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080905

(CHEMBL83681 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES COc1ccc(C[C@@H]2N(CC(=O)N[C@@H](CC3CCCN(C3)C(N)=N)C(=O)c3nccs3)C(=O)CN(CCCc3ccccc3)C2=O)cc1 Show InChI InChI=1S/C35H43N7O5S/c1-47-27-13-11-25(12-14-27)20-29-34(46)40(16-5-9-24-7-3-2-4-8-24)23-31(44)42(29)22-30(43)39-28(32(45)33-38-15-18-48-33)19-26-10-6-17-41(21-26)35(36)37/h2-4,7-8,11-15,18,26,28-29H,5-6,9-10,16-17,19-23H2,1H3,(H3,36,37)(H,39,43)/t26?,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289562

(CHEMBL3216390 | CHEMBL543774 | {(R)-1-Cyclohexylme...)Show SMILES Cl.Cl.NC(=N)NCCCNC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O |r| Show InChI InChI=1S/C21H38N6O4/c22-21(23)25-11-6-10-24-19(30)17-9-4-5-12-27(17)20(31)16(26-14-18(28)29)13-15-7-2-1-3-8-15/h15-17,26H,1-14H2,(H,24,30)(H,28,29)(H4,22,23,25)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Selective inhibition of thrombin in several in vitro and in vivo models of thrombosis |
Bioorg Med Chem Lett 7: 1563-1566 (1997)
Article DOI: 10.1016/S0960-894X(97)00271-0
BindingDB Entry DOI: 10.7270/Q27W6C68 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080913
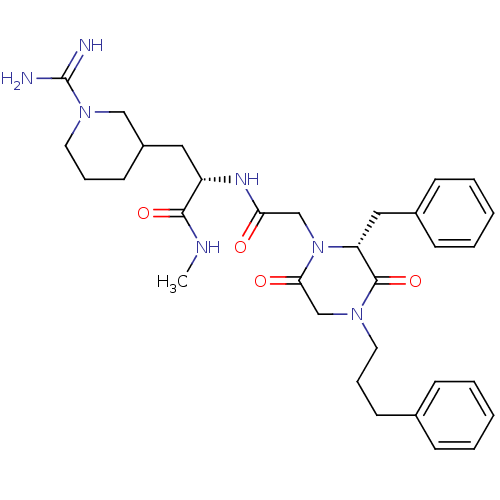
((S)-2-{2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propy...)Show SMILES CNC(=O)[C@H](CC1CCCN(C1)C(N)=N)NC(=O)CN1[C@H](Cc2ccccc2)C(=O)N(CCCc2ccccc2)CC1=O Show InChI InChI=1S/C32H43N7O4/c1-35-30(42)26(18-25-15-9-17-38(20-25)32(33)34)36-28(40)21-39-27(19-24-12-6-3-7-13-24)31(43)37(22-29(39)41)16-8-14-23-10-4-2-5-11-23/h2-7,10-13,25-27H,8-9,14-22H2,1H3,(H3,33,34)(H,35,42)(H,36,40)/t25?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080930

(2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...)Show SMILES [H][C@@]1(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)CCCN(C1)C(N)=N Show InChI InChI=1S/C34H41N7O4S/c35-34(36)40-17-8-14-26(21-40)19-27(31(44)32-37-15-18-46-32)38-29(42)22-41-28(20-25-11-5-2-6-12-25)33(45)39(23-30(41)43)16-7-13-24-9-3-1-4-10-24/h1-6,9-12,15,18,26-28H,7-8,13-14,16-17,19-23H2,(H3,35,36)(H,38,42)/t26?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080918

(CHEMBL408553 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccc4ccccc4c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C38H43N7O4S/c39-38(40)44-18-7-11-28(23-44)21-31(35(48)36-41-16-19-50-36)42-33(46)24-45-32(22-27-14-15-29-12-4-5-13-30(29)20-27)37(49)43(25-34(45)47)17-6-10-26-8-2-1-3-9-26/h1-5,8-9,12-16,19-20,28,31-32H,6-7,10-11,17-18,21-25H2,(H3,39,40)(H,42,46)/t28?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080896

(CHEMBL402843 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES [H][C@@]1(C[C@H](NC(=O)CN2[C@@H](C)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)CCCN(C1)C(N)=N Show InChI InChI=1S/C28H37N7O4S/c1-19-27(39)33(12-5-9-20-7-3-2-4-8-20)18-24(37)35(19)17-23(36)32-22(25(38)26-31-11-14-40-26)15-21-10-6-13-34(16-21)28(29)30/h2-4,7-8,11,14,19,21-22H,5-6,9-10,12-13,15-18H2,1H3,(H3,29,30)(H,32,36)/t19-,21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080907
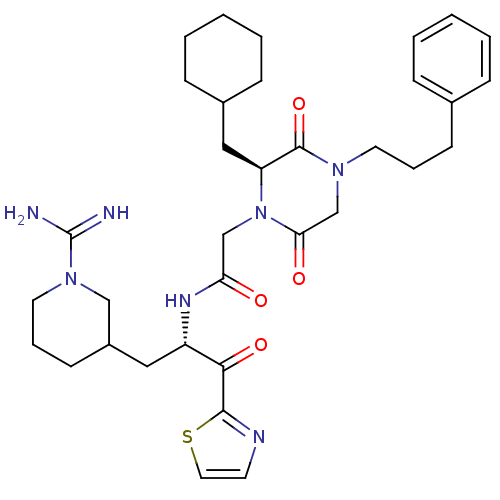
(CHEMBL311947 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](CC3CCCCC3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H47N7O4S/c35-34(36)40-17-8-14-26(21-40)19-27(31(44)32-37-15-18-46-32)38-29(42)22-41-28(20-25-11-5-2-6-12-25)33(45)39(23-30(41)43)16-7-13-24-9-3-1-4-10-24/h1,3-4,9-10,15,18,25-28H,2,5-8,11-14,16-17,19-23H2,(H3,35,36)(H,38,42)/t26?,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080917

(CHEMBL83001 | N-[(S)-2-Benzothiazol-2-yl-1-(1-carb...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nc3ccccc3s2)C1 Show InChI InChI=1S/C38H43N7O4S/c39-38(40)44-20-10-16-28(23-44)21-30(35(48)36-42-29-17-7-8-18-32(29)50-36)41-33(46)24-45-31(22-27-13-5-2-6-14-27)37(49)43(25-34(45)47)19-9-15-26-11-3-1-4-12-26/h1-8,11-14,17-18,28,30-31H,9-10,15-16,19-25H2,(H3,39,40)(H,41,46)/t28?,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080904
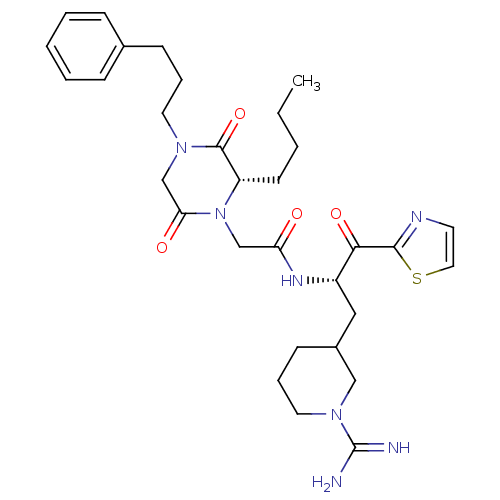
(2-[(S)-2-Butyl-3,6-dioxo-4-(3-phenyl-propyl)-piper...)Show SMILES CCCC[C@@H]1N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)C(=O)CN(CCCc2ccccc2)C1=O Show InChI InChI=1S/C31H43N7O4S/c1-2-3-13-25-30(42)36(15-7-11-22-9-5-4-6-10-22)21-27(40)38(25)20-26(39)35-24(28(41)29-34-14-17-43-29)18-23-12-8-16-37(19-23)31(32)33/h4-6,9-10,14,17,23-25H,2-3,7-8,11-13,15-16,18-21H2,1H3,(H3,32,33)(H,35,39)/t23?,24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080900
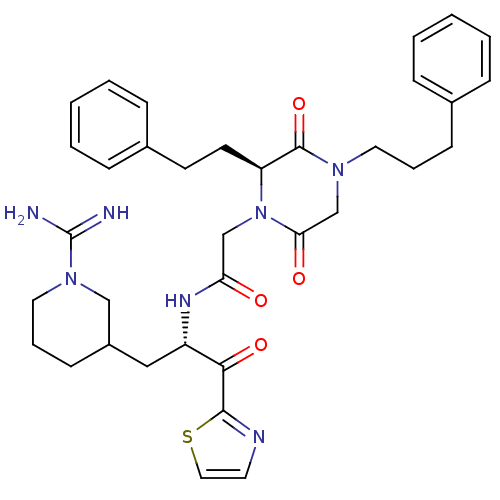
(CHEMBL84461 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](CCc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C35H43N7O4S/c36-35(37)41-19-8-14-27(22-41)21-28(32(45)33-38-17-20-47-33)39-30(43)23-42-29(16-15-26-11-5-2-6-12-26)34(46)40(24-31(42)44)18-7-13-25-9-3-1-4-10-25/h1-6,9-12,17,20,27-29H,7-8,13-16,18-19,21-24H2,(H3,36,37)(H,39,43)/t27?,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080914

(2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)N2CCCC2)C1 Show InChI InChI=1S/C35H47N7O4/c36-35(37)41-20-10-16-28(23-41)21-29(33(45)39-17-7-8-18-39)38-31(43)24-42-30(22-27-13-5-2-6-14-27)34(46)40(25-32(42)44)19-9-15-26-11-3-1-4-12-26/h1-6,11-14,28-30H,7-10,15-25H2,(H3,36,37)(H,38,43)/t28?,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080901

(CHEMBL84127 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES CN1CC(=O)N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)[C@@H](Cc2ccc3ccccc3c2)C1=O Show InChI InChI=1S/C30H35N7O4S/c1-35-18-26(39)37(24(29(35)41)15-19-8-9-21-6-2-3-7-22(21)13-19)17-25(38)34-23(27(40)28-33-10-12-42-28)14-20-5-4-11-36(16-20)30(31)32/h2-3,6-10,12-13,20,23-24H,4-5,11,14-18H2,1H3,(H3,31,32)(H,34,38)/t20?,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080906

(CHEMBL316383 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES CC(C)C[C@@H]1N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)C(=O)CN(CCCc2ccccc2)C1=O Show InChI InChI=1S/C31H43N7O4S/c1-21(2)16-25-30(42)36(13-6-10-22-8-4-3-5-9-22)20-27(40)38(25)19-26(39)35-24(28(41)29-34-12-15-43-29)17-23-11-7-14-37(18-23)31(32)33/h3-5,8-9,12,15,21,23-25H,6-7,10-11,13-14,16-20H2,1-2H3,(H3,32,33)(H,35,39)/t23?,24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080898
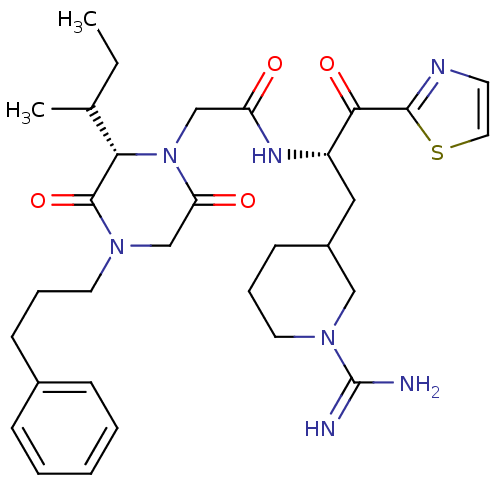
(2-[(S)-2-sec-Butyl-3,6-dioxo-4-(3-phenyl-propyl)-p...)Show SMILES CCC(C)[C@@H]1N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)C(=O)CN(CCCc2ccccc2)C1=O Show InChI InChI=1S/C31H43N7O4S/c1-3-21(2)27-30(42)36(14-7-11-22-9-5-4-6-10-22)20-26(40)38(27)19-25(39)35-24(28(41)29-34-13-16-43-29)17-23-12-8-15-37(18-23)31(32)33/h4-6,9-10,13,16,21,23-24,27H,3,7-8,11-12,14-15,17-20H2,1-2H3,(H3,32,33)(H,35,39)/t21?,23?,24-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080899

(CHEMBL309931 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2CC(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C27H35N7O4S/c28-27(29)33-12-5-9-20(15-33)14-21(25(38)26-30-10-13-39-26)31-22(35)16-34-18-23(36)32(17-24(34)37)11-4-8-19-6-2-1-3-7-19/h1-3,6-7,10,13,20-21H,4-5,8-9,11-12,14-18H2,(H3,28,29)(H,31,35)/t20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 495 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2497-502 (1999)
BindingDB Entry DOI: 10.7270/Q23T9GDD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080912
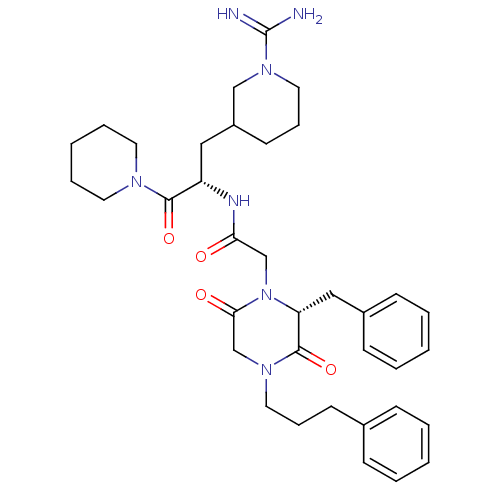
(2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)N2CCCCC2)C1 Show InChI InChI=1S/C36H49N7O4/c37-36(38)42-21-11-17-29(24-42)22-30(34(46)40-18-8-3-9-19-40)39-32(44)25-43-31(23-28-14-6-2-7-15-28)35(47)41(26-33(43)45)20-10-16-27-12-4-1-5-13-27/h1-2,4-7,12-15,29-31H,3,8-11,16-26H2,(H3,37,38)(H,39,44)/t29?,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 825 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50601510
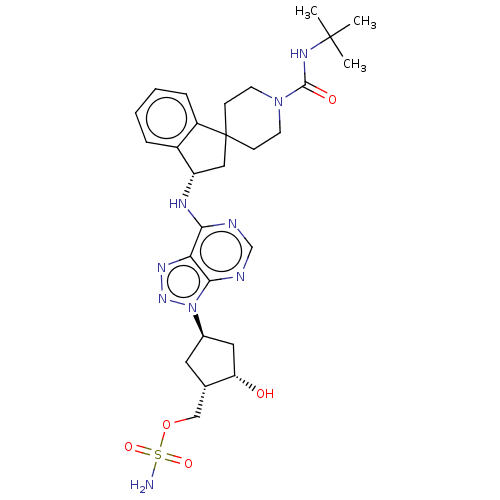
(CHEMBL5170838)Show SMILES CC(C)(C)NC(=O)N1CCC2(C[C@H](Nc3ncnc4n(nnc34)[C@H]3C[C@H](O)[C@H](COS(N)(=O)=O)C3)c3ccccc23)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin A receptor in cultured rabbit renal artery vascular smooth muscle cells |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50601479
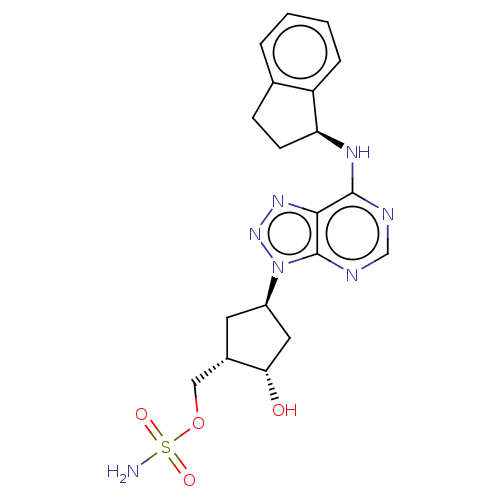
(CHEMBL5182746)Show SMILES NS(=O)(=O)OC[C@@H]1C[C@H](C[C@@H]1O)n1nnc2c(N[C@H]3CCc4ccccc34)ncnc12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50285607
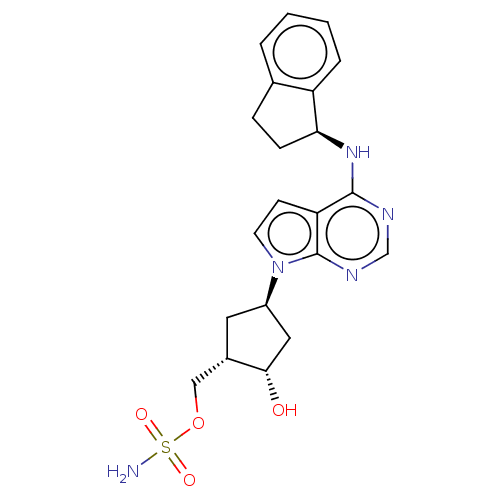
(MLN-4924 | MLN-4924003 | Pevonedistat)Show SMILES [H][C@]1(O)C[C@@]([H])(C[C@@]1([H])COS(N)(=O)=O)n1ccc2c(N[C@@]3([H])CCc4ccccc34)ncnc12 |r| Show InChI InChI=1S/C21H25N5O4S/c22-31(28,29)30-11-14-9-15(10-19(14)27)26-8-7-17-20(23-12-24-21(17)26)25-18-6-5-13-3-1-2-4-16(13)18/h1-4,7-8,12,14-15,18-19,27H,5-6,9-11H2,(H2,22,28,29)(H,23,24,25)/t14-,15+,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50427934
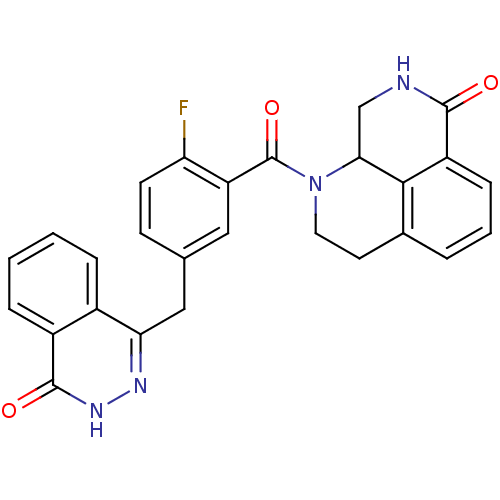
(CHEMBL2322618)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C27H21FN4O3/c28-21-9-8-15(13-22-17-5-1-2-6-18(17)26(34)31-30-22)12-20(21)27(35)32-11-10-16-4-3-7-19-24(16)23(32)14-29-25(19)33/h1-9,12,23H,10-11,13-14H2,(H,29,33)(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 after 1 hr by ELISA |
J Med Chem 56: 2885-903 (2013)
Article DOI: 10.1021/jm301825t
BindingDB Entry DOI: 10.7270/Q26M385C |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048114
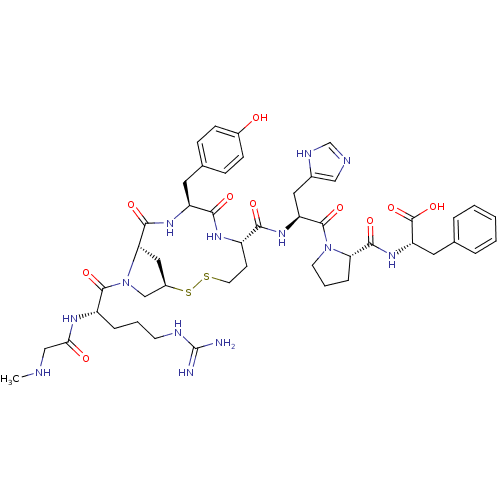
(CHEMBL384352 | c[Sar1-Arg2-Mpt3-Tyr4-Hcy5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N1C[C@H]2C[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCSS2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O10S2/c1-50-24-39(62)54-33(9-5-16-52-47(48)49)44(67)60-25-31-22-38(60)43(66)56-34(19-28-11-13-30(61)14-12-28)41(64)55-32(15-18-71-72-31)40(63)57-35(21-29-23-51-26-53-29)45(68)59-17-6-10-37(59)42(65)58-36(46(69)70)20-27-7-3-2-4-8-27/h2-4,7-8,11-14,23,26,31-38,50,61H,5-6,9-10,15-22,24-25H2,1H3,(H,51,53)(H,54,62)(H,55,64)(H,56,66)(H,57,63)(H,58,65)(H,69,70)(H4,48,49,52)/t31-,32+,33+,34+,35+,36+,37+,38+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50594972
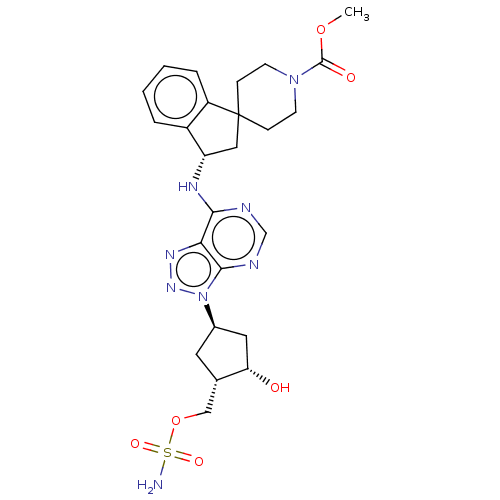
(CHEMBL5177755)Show SMILES COC(=O)N1CCC2(C[C@H](Nc3ncnc4n(nnc34)[C@H]3C[C@H](O)[C@H](COS(N)(=O)=O)C3)c3ccccc23)CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50601508
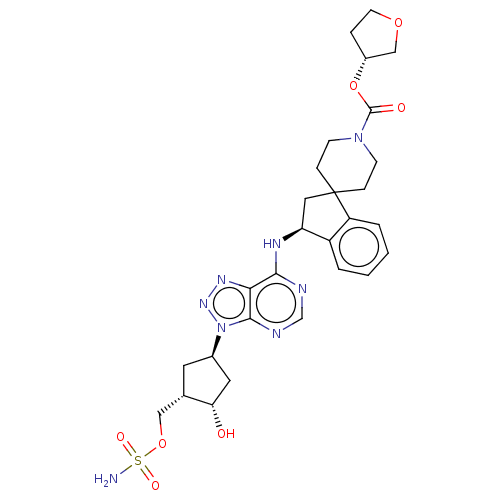
(CHEMBL5206652)Show SMILES NS(=O)(=O)OC[C@@H]1C[C@H](C[C@@H]1O)n1nnc2c(N[C@H]3CC4(CCN(CC4)C(=O)O[C@@H]4CCOC4)c4ccccc34)ncnc12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048119
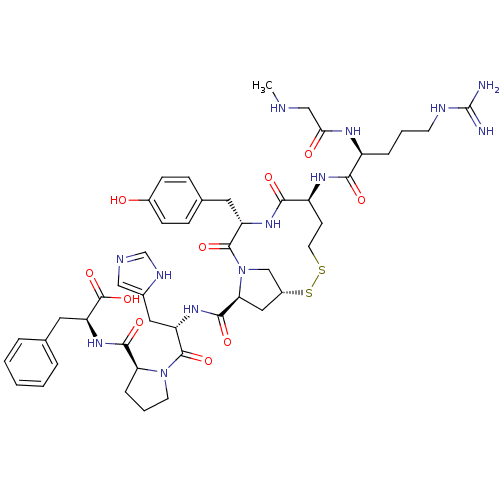
(CHEMBL385283 | c[Sar1-Arg2-Hcy3-Tyr4-MPt5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSS[C@@H]2C[C@H](N(C2)C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O10S2/c1-50-24-39(62)54-32(9-5-16-52-47(48)49)40(63)55-33-15-18-71-72-31-22-38(60(25-31)45(68)34(56-41(33)64)19-28-11-13-30(61)14-12-28)43(66)57-35(21-29-23-51-26-53-29)44(67)59-17-6-10-37(59)42(65)58-36(46(69)70)20-27-7-3-2-4-8-27/h2-4,7-8,11-14,23,26,31-38,50,61H,5-6,9-10,15-22,24-25H2,1H3,(H,51,53)(H,54,62)(H,55,63)(H,56,64)(H,57,66)(H,58,65)(H,69,70)(H4,48,49,52)/t31-,32+,33+,34+,35+,36+,37+,38+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048113
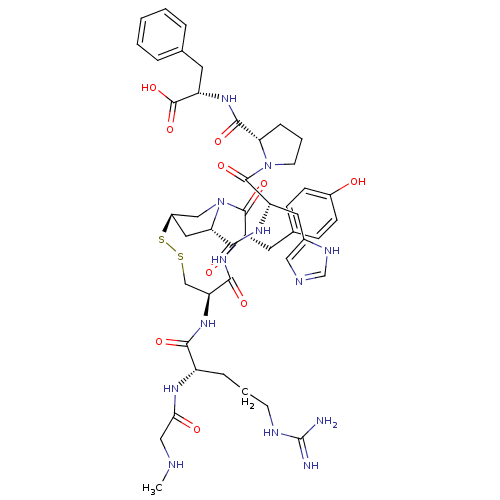
(CHEMBL414533 | c[Sar1-Arg2-Cys3-Tyr4-MPt5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSS[C@@H]2C[C@H](N(C2)C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C46H61N13O10S2/c1-49-22-38(61)53-31(9-5-15-51-46(47)48)39(62)57-35-24-70-71-30-20-37(59(23-30)44(67)32(54-40(35)63)17-27-11-13-29(60)14-12-27)42(65)55-33(19-28-21-50-25-52-28)43(66)58-16-6-10-36(58)41(64)56-34(45(68)69)18-26-7-3-2-4-8-26/h2-4,7-8,11-14,21,25,30-37,49,60H,5-6,9-10,15-20,22-24H2,1H3,(H,50,52)(H,53,61)(H,54,63)(H,55,65)(H,56,64)(H,57,62)(H,68,69)(H4,47,48,51)/t30-,31+,32+,33+,34+,35+,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369268
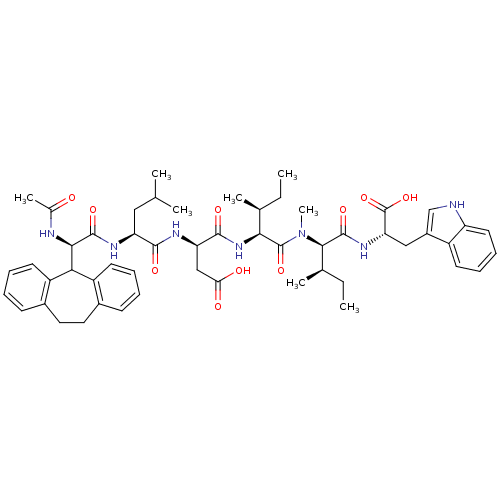
(CHEMBL1790519)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)C(=O)N(C)[C@H]([C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C53H69N7O10/c1-9-30(5)45(52(68)60(8)47(31(6)10-2)51(67)58-42(53(69)70)26-35-28-54-39-22-16-15-19-36(35)39)59-49(65)41(27-43(62)63)56-48(64)40(25-29(3)4)57-50(66)46(55-32(7)61)44-37-20-13-11-17-33(37)23-24-34-18-12-14-21-38(34)44/h11-22,28-31,40-42,44-47,54H,9-10,23-27H2,1-8H3,(H,55,61)(H,56,64)(H,57,66)(H,58,67)(H,59,65)(H,62,63)(H,69,70)/t30-,31+,40-,41+,42-,45-,46+,47+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
NEDD8-activating enzyme E1 catalytic subunit
(Homo sapiens) | BDBM50601511
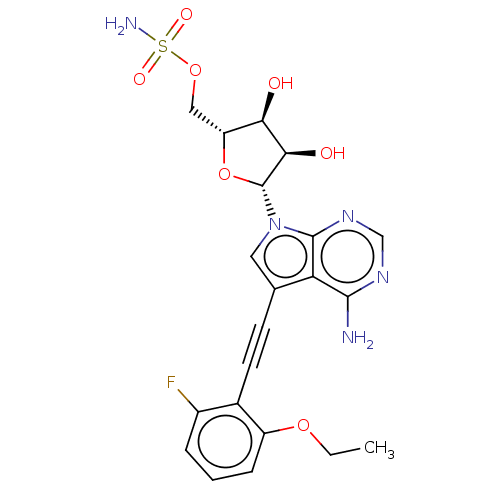
(CHEMBL5181979)Show SMILES CCOc1cccc(F)c1C#Cc1cn([C@@H]2O[C@H](COS(N)(=O)=O)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00242
BindingDB Entry DOI: 10.7270/Q2HX1HQK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity to the angiotensin II receptor, type 1 in rat liver |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072741
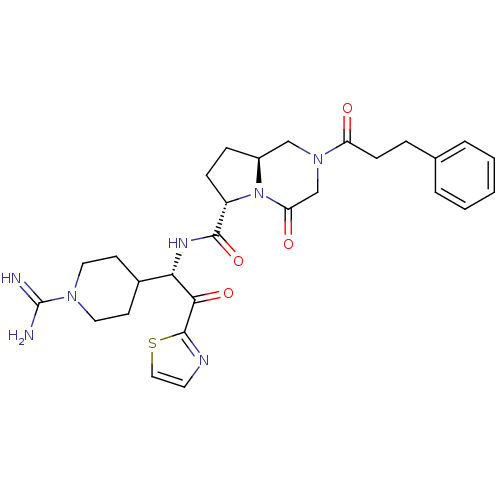
((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...)Show SMILES NC(=N)N1CCC(CC1)[C@H](NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C28H35N7O4S/c29-28(30)33-13-10-19(11-14-33)24(25(38)27-31-12-15-40-27)32-26(39)21-8-7-20-16-34(17-23(37)35(20)21)22(36)9-6-18-4-2-1-3-5-18/h1-5,12,15,19-21,24H,6-11,13-14,16-17H2,(H3,29,30)(H,32,39)/t20-,21-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against thrombin(FIIa) |
Bioorg Med Chem Lett 8: 3409-14 (1999)
BindingDB Entry DOI: 10.7270/Q27943VV |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072740
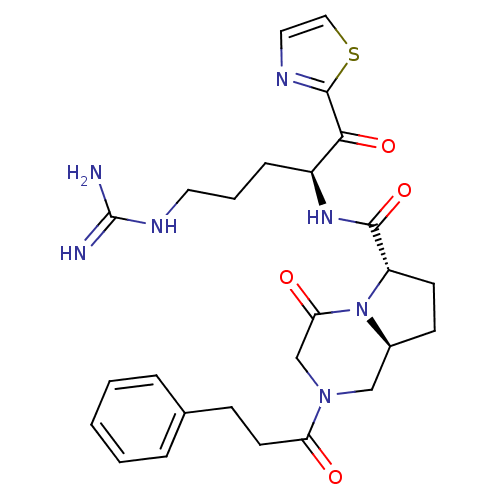
((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O4S/c27-26(28)30-12-4-7-19(23(36)25-29-13-14-38-25)31-24(37)20-10-9-18-15-32(16-22(35)33(18)20)21(34)11-8-17-5-2-1-3-6-17/h1-3,5-6,13-14,18-20H,4,7-12,15-16H2,(H,31,37)(H4,27,28,30)/t18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against thrombin(FIIa) |
Bioorg Med Chem Lett 8: 3409-14 (1999)
BindingDB Entry DOI: 10.7270/Q27943VV |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50286614
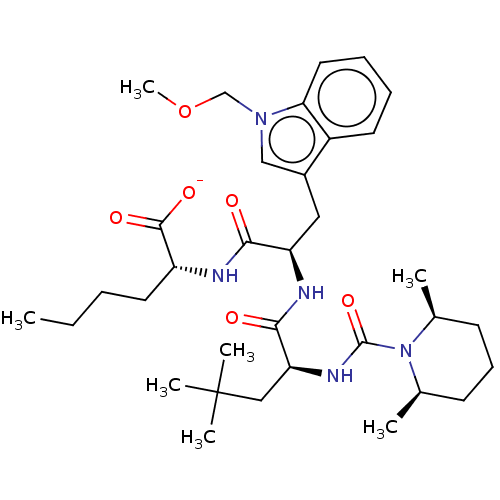
(CHEMBL155943 | CHEMBL3143473 | Sodium; 2-[2-{2-[(2...)Show SMILES [Na+].CCCC[C@@H](NC(=O)[C@@H](Cc1cn(COC)c2ccccc12)NC(=O)[C@H](CC(C)(C)C)NC(=O)N1[C@@H](C)CCC[C@H]1C)C([O-])=O |r| Show InChI InChI=1S/C34H53N5O6/c1-8-9-16-26(32(42)43)35-30(40)27(18-24-20-38(21-45-7)29-17-11-10-15-25(24)29)36-31(41)28(19-34(4,5)6)37-33(44)39-22(2)13-12-14-23(39)3/h10-11,15,17,20,22-23,26-28H,8-9,12-14,16,18-19,21H2,1-7H3,(H,35,40)(H,36,41)(H,37,44)(H,42,43)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against endothelin B (ETB) receptor using human girardi heart cells. |
Bioorg Med Chem Lett 5: 621-626 (1995)
Article DOI: 10.1016/0960-894X(95)00084-7
BindingDB Entry DOI: 10.7270/Q27081DM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50048118
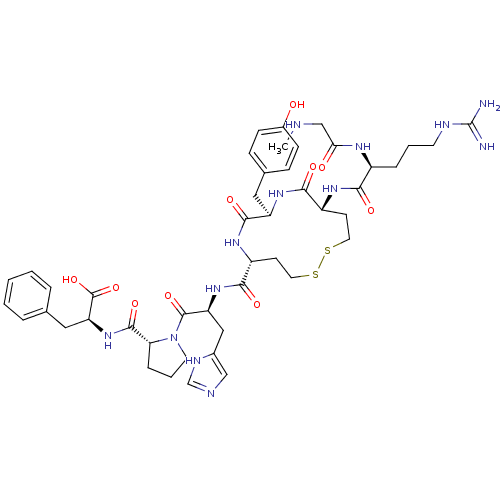
(2-({1-[2-{[11-[5-Guanidino-2-(2-methylamino-acetyl...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CCSSCC[C@@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C46H63N13O10S2/c1-49-25-38(61)53-31(9-5-17-51-46(47)48)39(62)54-32-15-19-70-71-20-16-33(55-42(65)34(56-40(32)63)21-28-11-13-30(60)14-12-28)41(64)57-35(23-29-24-50-26-52-29)44(67)59-18-6-10-37(59)43(66)58-36(45(68)69)22-27-7-3-2-4-8-27/h2-4,7-8,11-14,24,26,31-37,49,60H,5-6,9-10,15-23,25H2,1H3,(H,50,52)(H,53,61)(H,54,62)(H,55,65)(H,56,63)(H,57,64)(H,58,66)(H,68,69)(H4,47,48,51)/t31-,32+,33+,34+,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity to the angiotensin II receptor, type 1 in rat liver |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50048119

(CHEMBL385283 | c[Sar1-Arg2-Hcy3-Tyr4-MPt5-His6-Pro...)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSS[C@@H]2C[C@H](N(C2)C(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O10S2/c1-50-24-39(62)54-32(9-5-16-52-47(48)49)40(63)55-33-15-18-71-72-31-22-38(60(25-31)45(68)34(56-41(33)64)19-28-11-13-30(61)14-12-28)43(66)57-35(21-29-23-51-26-53-29)44(67)59-17-6-10-37(59)42(65)58-36(46(69)70)20-27-7-3-2-4-8-27/h2-4,7-8,11-14,23,26,31-38,50,61H,5-6,9-10,15-22,24-25H2,1H3,(H,51,53)(H,54,62)(H,55,63)(H,56,64)(H,57,66)(H,58,65)(H,69,70)(H4,48,49,52)/t31-,32+,33+,34+,35+,36+,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity to the angiotensin II receptor, type 1 in rat liver |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
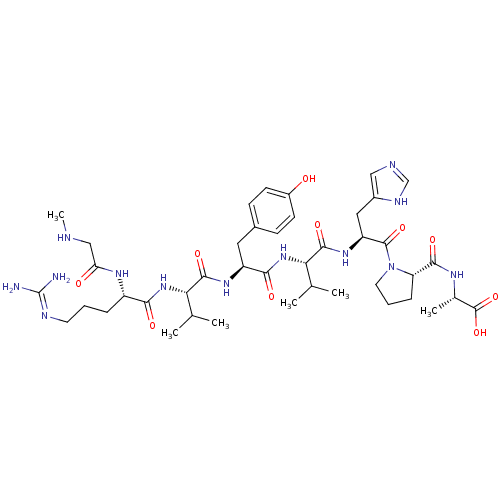
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50531806
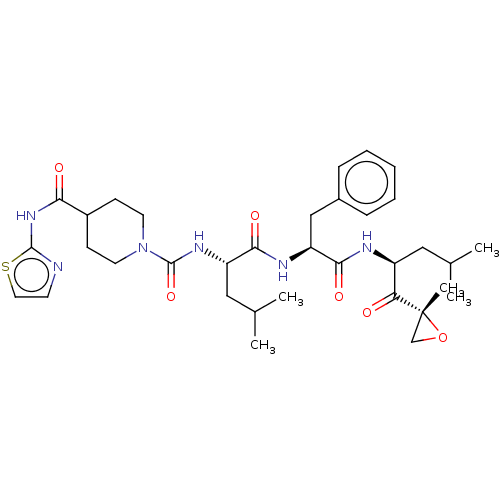
(CHEMBL4460323)Show SMILES CC(C)C[C@H](NC(=O)N1CCC(CC1)C(=O)Nc1nccs1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C34H48N6O6S/c1-21(2)17-25(28(41)34(5)20-46-34)36-31(44)27(19-23-9-7-6-8-10-23)37-30(43)26(18-22(3)4)38-33(45)40-14-11-24(12-15-40)29(42)39-32-35-13-16-47-32/h6-10,13,16,21-22,24-27H,11-12,14-15,17-20H2,1-5H3,(H,36,44)(H,37,43)(H,38,45)(H,35,39,42)/t25-,26-,27-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human 20s constitutive proteasome beta5 chymotrypsin-like activity using Suc-LLVY-AMC as substrate preincubated for 15 mins followed by... |
Eur J Med Chem 164: 602-614 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.064
BindingDB Entry DOI: 10.7270/Q2JM2F38 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50531806

(CHEMBL4460323)Show SMILES CC(C)C[C@H](NC(=O)N1CCC(CC1)C(=O)Nc1nccs1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C34H48N6O6S/c1-21(2)17-25(28(41)34(5)20-46-34)36-31(44)27(19-23-9-7-6-8-10-23)37-30(43)26(18-22(3)4)38-33(45)40-14-11-24(12-15-40)29(42)39-32-35-13-16-47-32/h6-10,13,16,21-22,24-27H,11-12,14-15,17-20H2,1-5H3,(H,36,44)(H,37,43)(H,38,45)(H,35,39,42)/t25-,26-,27-,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition |
Eur J Med Chem 164: 602-614 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.064
BindingDB Entry DOI: 10.7270/Q2JM2F38 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin B receptor in cultured rat cerebellar membranes |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50048127

(Angiotensin III | CHEMBL56448)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C40H60N12O11/c1-20(2)31(50-34(57)26(7-5-13-45-40(42)43)47-33(56)25(41)17-30(54)55)36(59)48-27(15-22-9-11-24(53)12-10-22)35(58)51-32(21(3)4)37(60)49-28(16-23-18-44-19-46-23)38(61)52-14-6-8-29(52)39(62)63/h9-12,18-21,25-29,31-32,53H,5-8,13-17,41H2,1-4H3,(H,44,46)(H,47,56)(H,48,59)(H,49,60)(H,50,57)(H,51,58)(H,54,55)(H,62,63)(H4,42,43,45)/t25-,26-,27-,28-,29-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro binding affinity at angiotensin II (type 2) receptor in rabbit uterus. |
J Med Chem 36: 1902-13 (1993)
BindingDB Entry DOI: 10.7270/Q2N58KF4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data