

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007477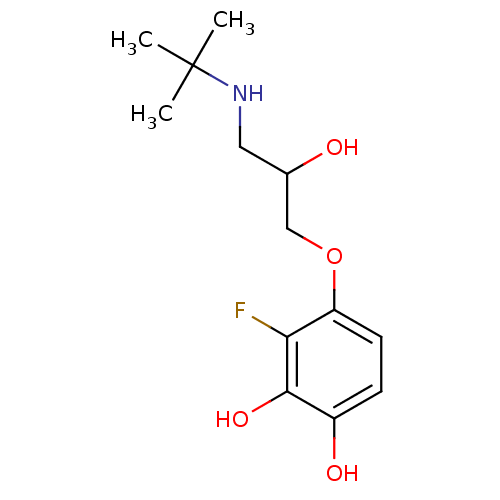 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-3-fluoro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-dihydroalprenolol from beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007476 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-benzene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-dihydroalprenolol from beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-dihydroalprenolol from beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50279966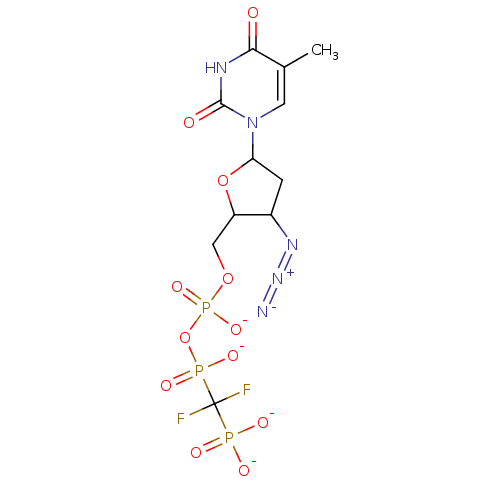 (Azidothymidine difluoromethylenephosphonate deriva...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was determined in an HIV-1 reverse transcriptase assay in which the [3H]dTTP concentration was varied (i.e. 40, 20, 10, 6, and 4 ... | Bioorg Med Chem Lett 1: 357-360 (1991) Article DOI: 10.1016/S0960-894X(01)80472-8 BindingDB Entry DOI: 10.7270/Q2PV6KVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007478 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-5-fluoro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-dihydroalprenolol from beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration that causes 50 % inhibition of HIV-1 peptide-derived reverse transcriptase (RT) obtained from HIV-1-infected H9 cell cultures | Bioorg Med Chem Lett 1: 357-360 (1991) Article DOI: 10.1016/S0960-894X(01)80472-8 BindingDB Entry DOI: 10.7270/Q2PV6KVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50279966 (Azidothymidine difluoromethylenephosphonate deriva...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration that causes 50 % inhibition of HIV-1 peptide-derived reverse transcriptase (RT) | Bioorg Med Chem Lett 1: 357-360 (1991) Article DOI: 10.1016/S0960-894X(01)80472-8 BindingDB Entry DOI: 10.7270/Q2PV6KVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50279965 (Azidothymidine difluoromethylenephosphonate deriva...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration that causes 50 % inhibition of HIV-1 peptide-derived reverse transcriptase (RT) | Bioorg Med Chem Lett 1: 357-360 (1991) Article DOI: 10.1016/S0960-894X(01)80472-8 BindingDB Entry DOI: 10.7270/Q2PV6KVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Beta adrenergic receptor agonistic activity for the stimulation of accumulation of cyclic AMP in cultured C6 glioma cells | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007476 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-benzene-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Beta adrenergic receptor agonistic activity for the stimulation of accumulation of cyclic AMP in cultured C6 glioma cells | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50007477 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-3-fluoro-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Activity was evaluated by measuring the inhibition of isolated hog H+/K+ ATPase | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50007477 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-3-fluoro-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta2- adrenergic) for the percent maximal relaxation of isolated guinea pig trachea | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (GUINEA PIG) | BDBM50007477 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-3-fluoro-b...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta-1 adrenergic receptor) for the percent maximal increase in contraction rate of isolated guinea pig atria | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50007476 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-benzene-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta2- adrenergic) for the percent maximal relaxation of isolated guinea pig trachea | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta2- adrenergic) for the percent maximal relaxation of isolated guinea pig trachea | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (GUINEA PIG) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta-1 adrenergic receptor) for the percent maximal increase in contraction rate of isolated guinea pig atria | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (GUINEA PIG) | BDBM50007476 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-benzene-1,...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta-1 adrenergic receptor) for the percent maximal increase in contraction rate of isolated guinea pig atria | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (GUINEA PIG) | BDBM50007478 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-5-fluoro-b...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 175 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonistic activity (beta-1 adrenergic receptor) for the percent maximal increase in contraction rate of isolated guinea pig atria | J Med Chem 34: 1063-8 (1991) BindingDB Entry DOI: 10.7270/Q2765D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||