

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407675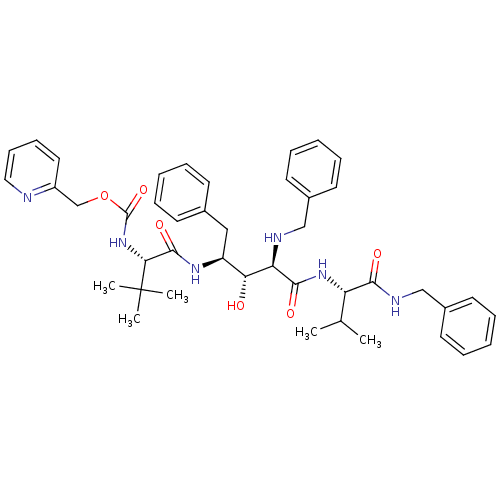 (CHEMBL342293) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1226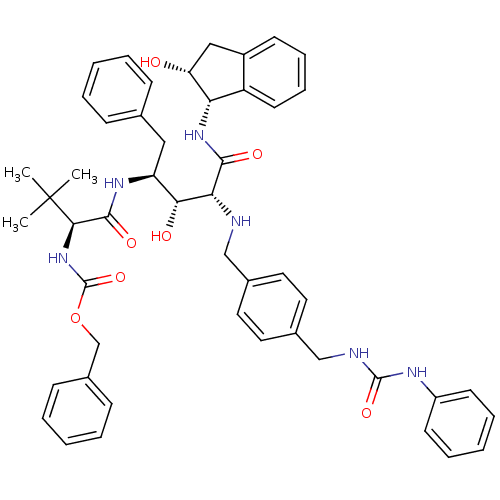 (2-Aminobenzyl-Substituted AHPPA deriv. 44 | benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407702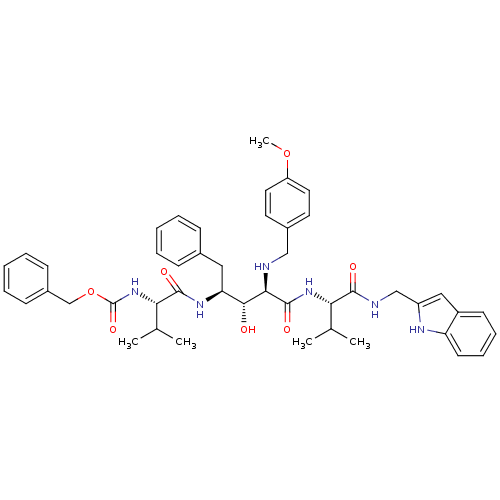 (CHEMBL357477) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM874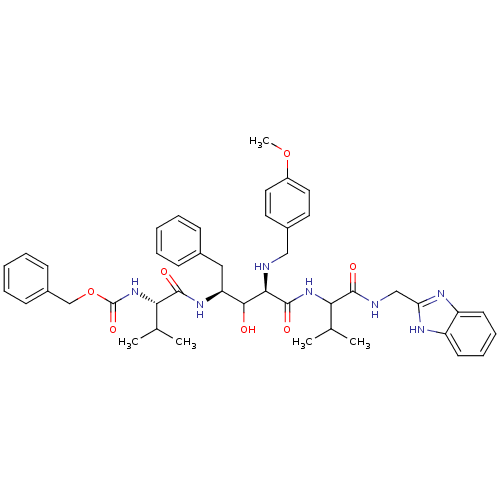 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 1 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -50.3 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1228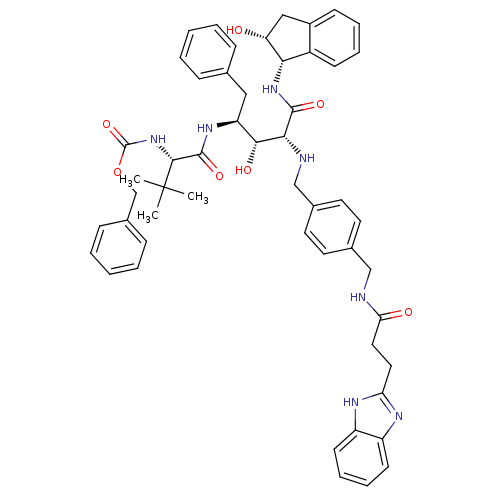 (2-Aminobenzyl-Substituted AHPPA deriv. 46 | benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407660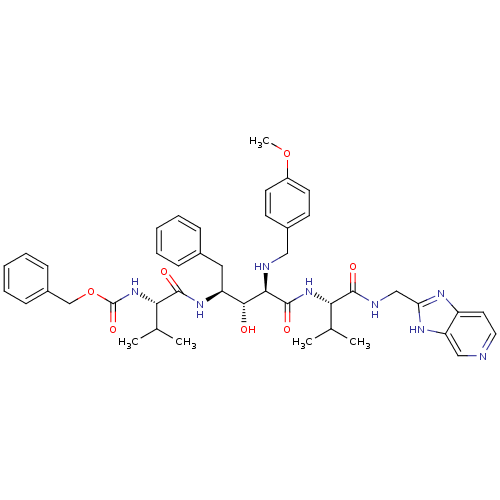 (CHEMBL342319) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407703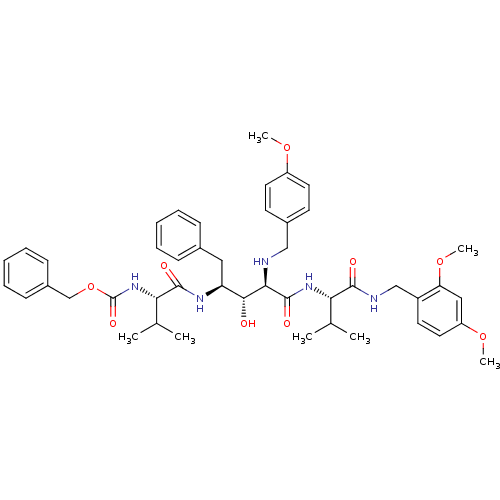 (CHEMBL25374) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407681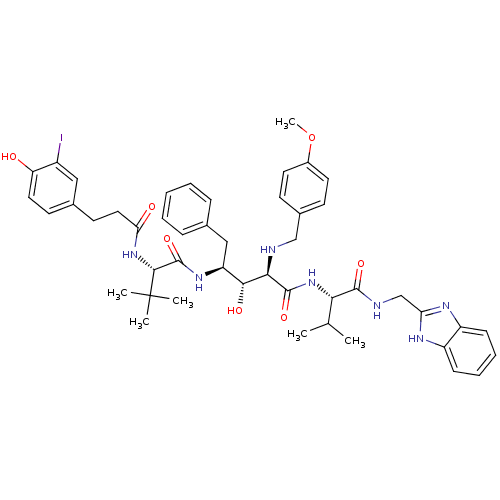 (CHEMBL356941) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM967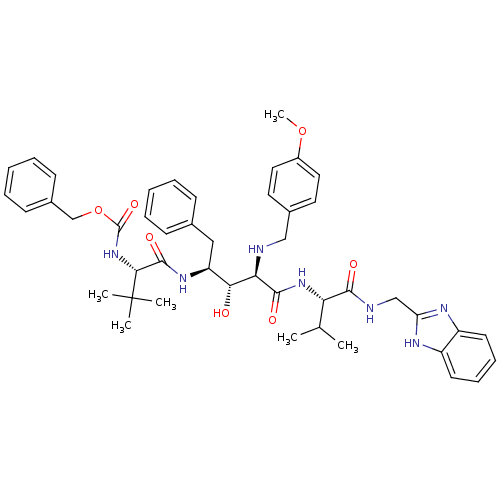 (5PhBuCOOH deriv. | Statine deriv. 50 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407650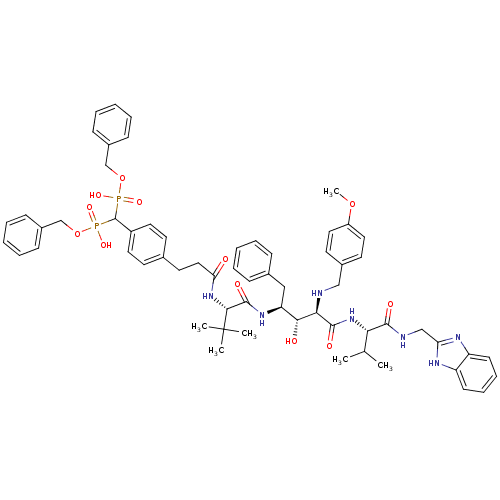 (CHEMBL263779) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407695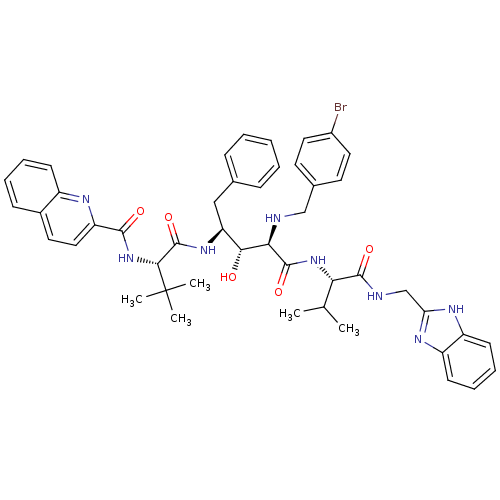 (CHEMBL357894) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1225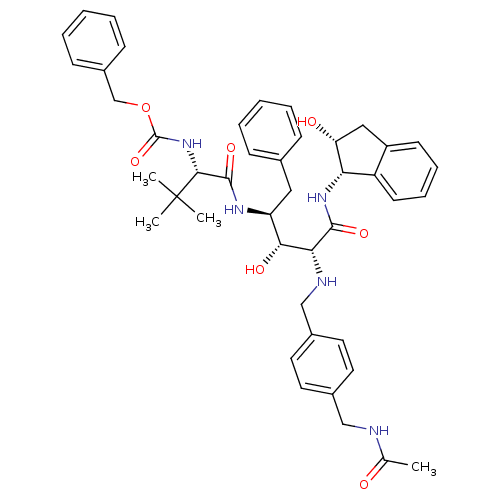 (2-Aminobenzyl-Substituted AHPPA deriv. 43 | benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407667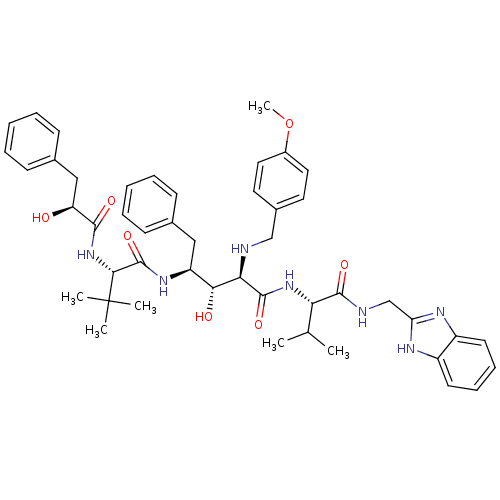 (CHEMBL344977) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407652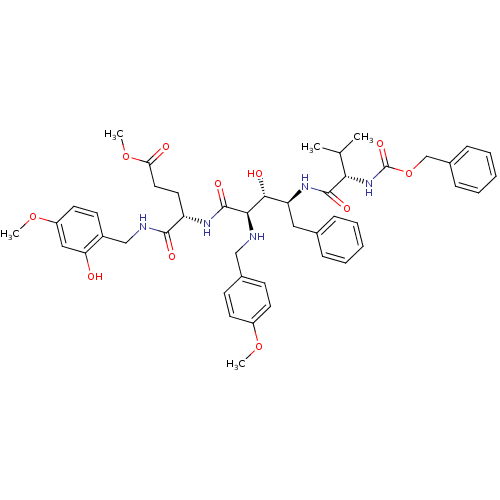 (CHEMBL147904) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM926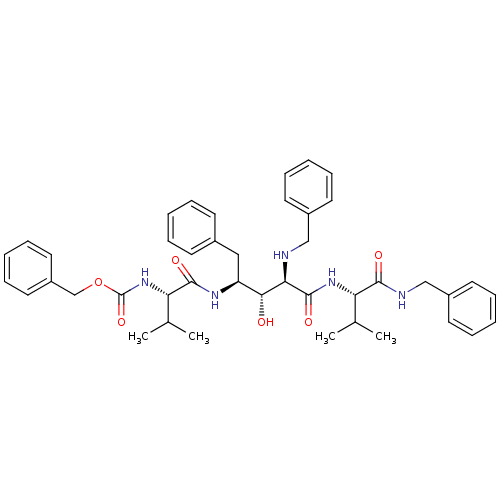 (5PhBuCOOH deriv. | Statine deriv. 6 | Tle-Val-Sta ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM965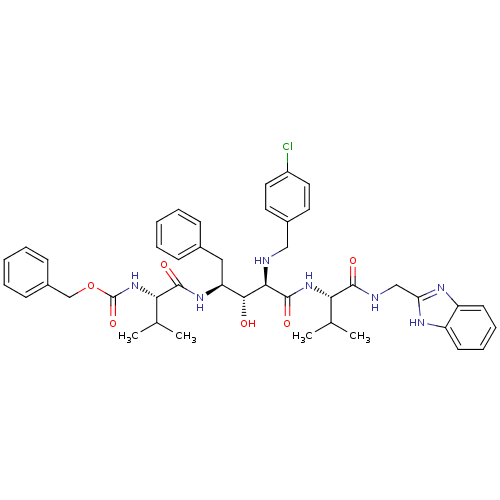 (5PhBuCOOH deriv. | Statine deriv. 48 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407645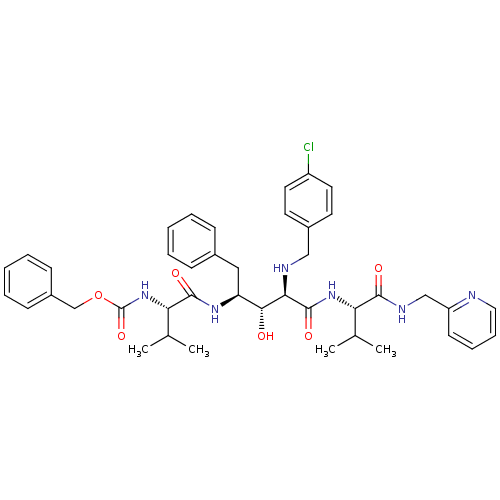 (CHEMBL342067) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM856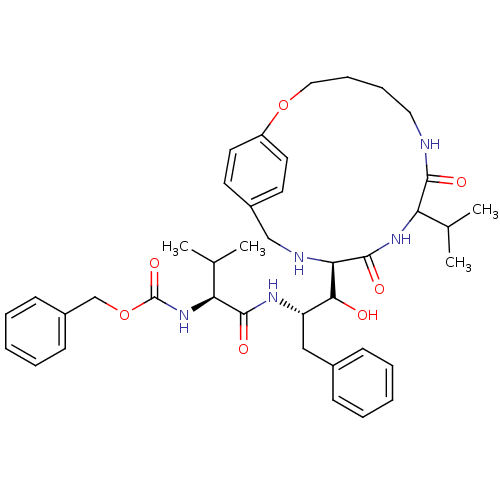 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 22 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -48.6 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM858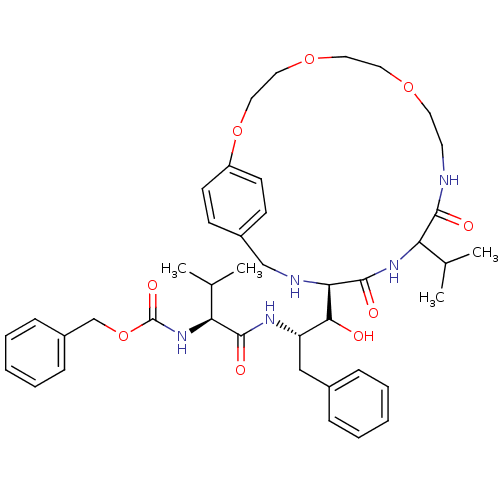 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 23 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90 | -48.5 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM928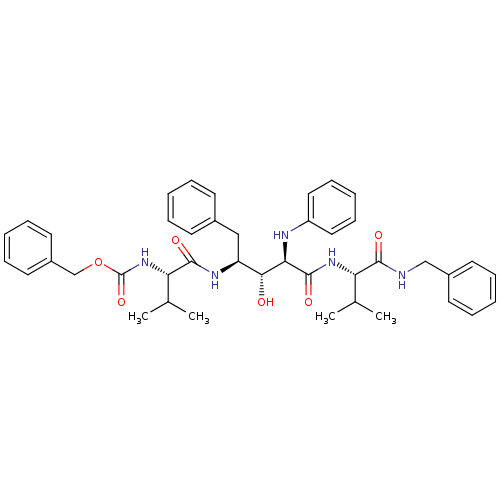 (5PhBuCOOH deriv. | Statine deriv. 8 | Tle-Val-Sta ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407643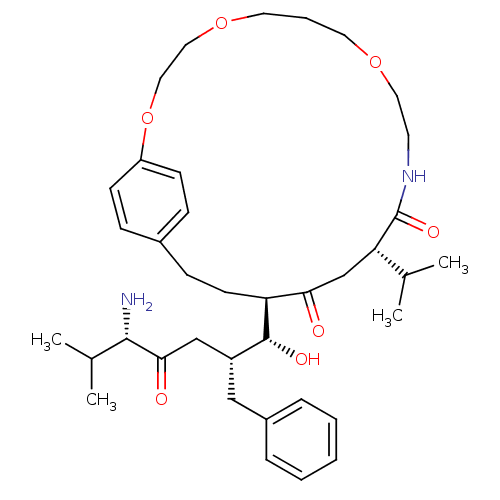 (CHEMBL357218) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407676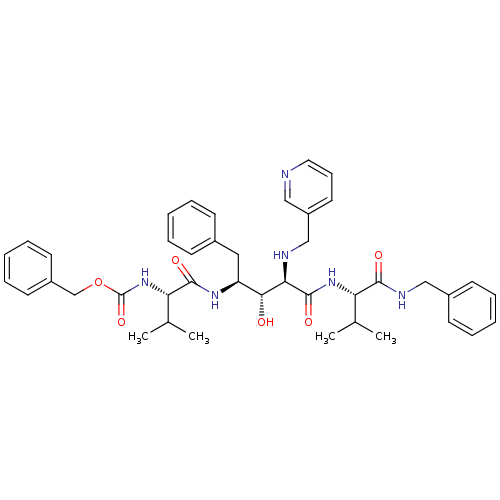 (CHEMBL341746) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM936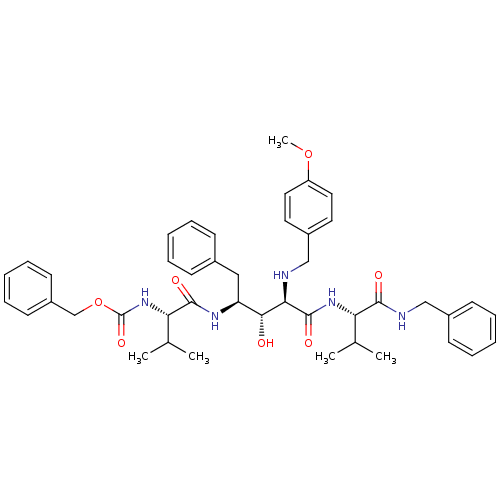 (5PhBuCOOH deriv. | Statine deriv. 16 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM859 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 24 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407646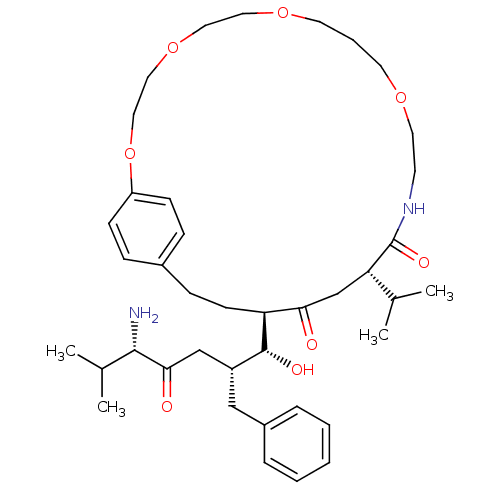 (CHEMBL147096) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM966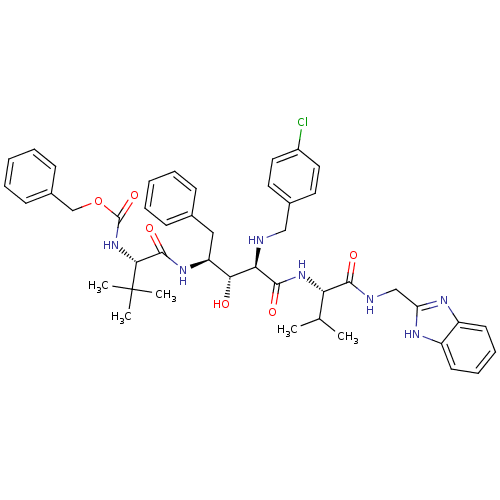 (5PhBuCOOH deriv. | Statine deriv. 49 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM865 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 20 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.60 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM866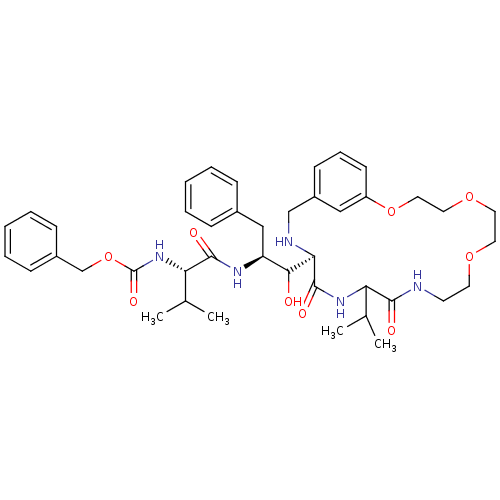 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 29 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.70 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407698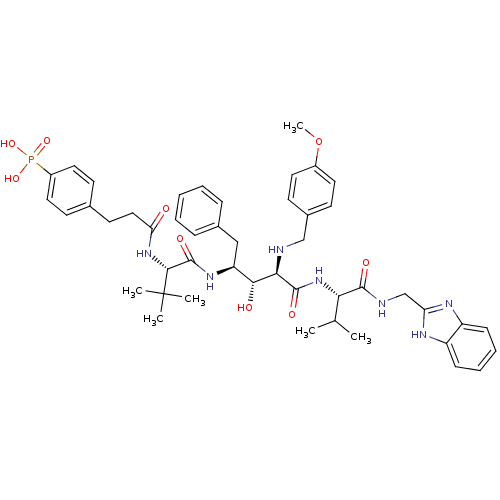 (CHEMBL144405) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407658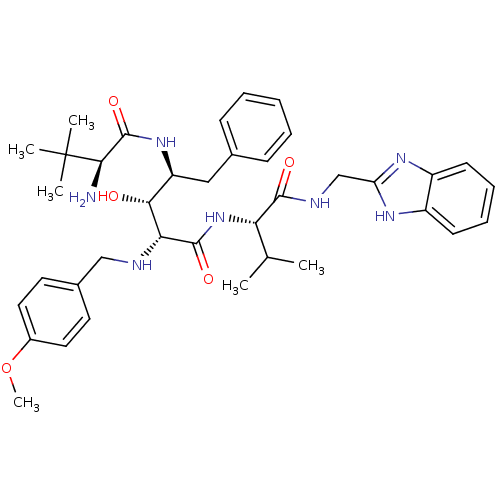 (CHEMBL357846) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1197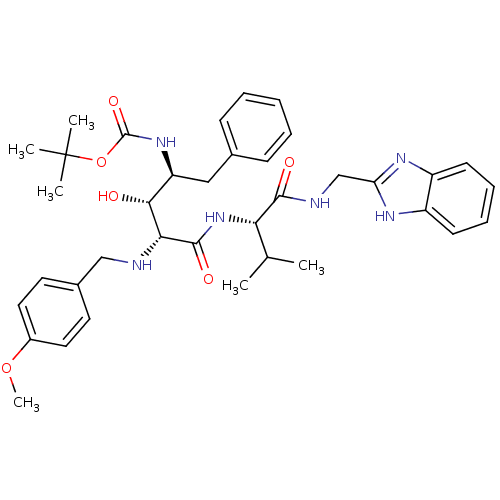 (2-Aminobenzyl-Substituted AHPPA deriv. 12 | tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM857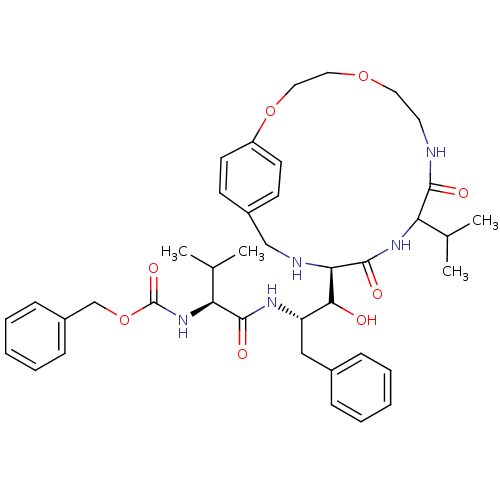 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 3 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | -48.1 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM870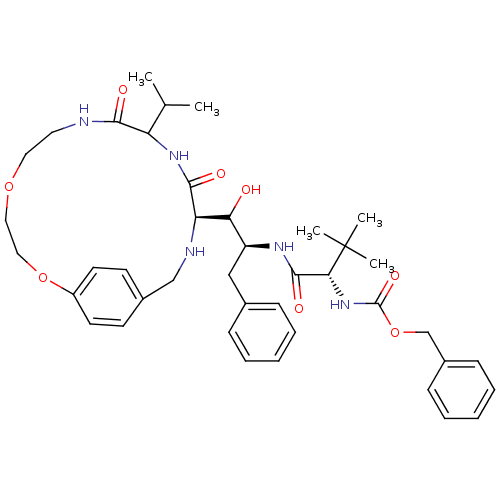 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 32 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM872 ((3R)-oxolan-3-yl N-[(1S,2S)-1-[(10R,13R)-9,12-diox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407687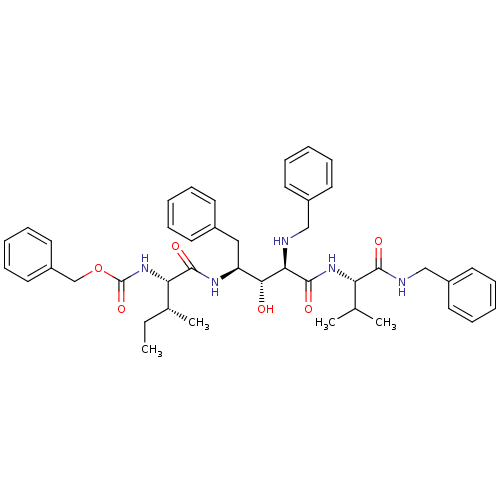 (CHEMBL1790800) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM954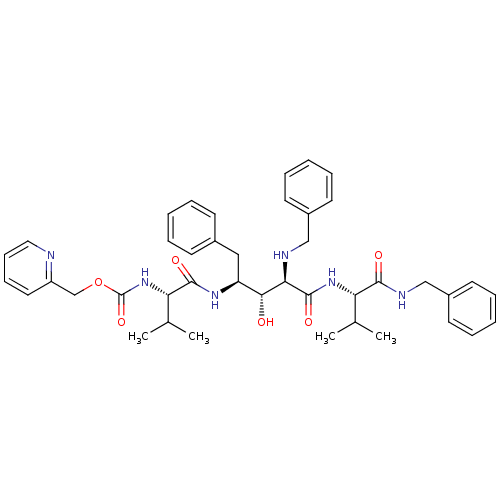 (5PhBuCOOH deriv. | Statine deriv. 37 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407683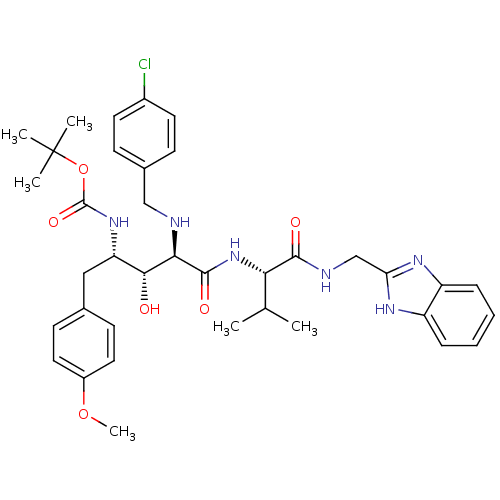 (CHEMBL145701) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407654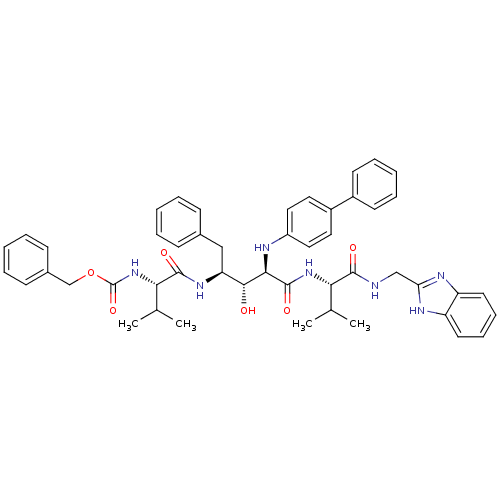 (CHEMBL146890) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407629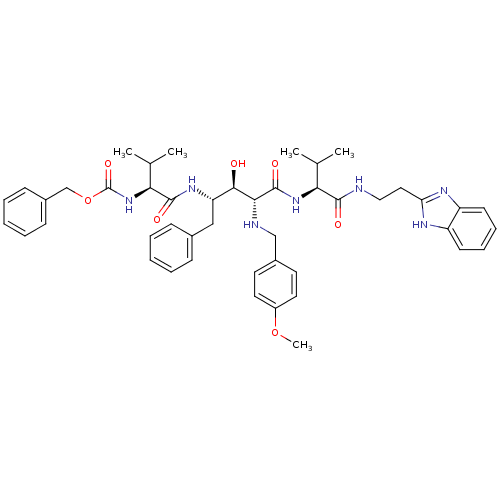 (CHEMBL343440) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM942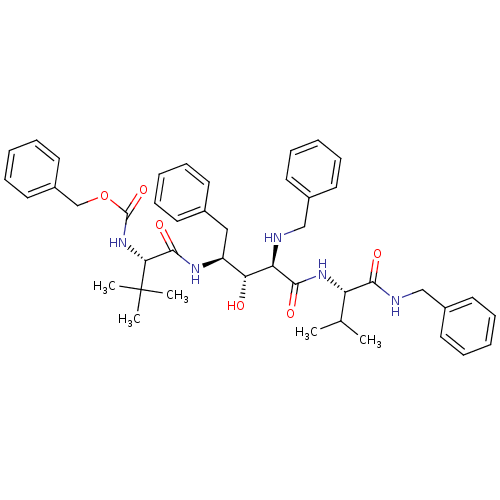 (5PhBuCOOH deriv. | Statine deriv. 22 | Tle-Val-Sta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM864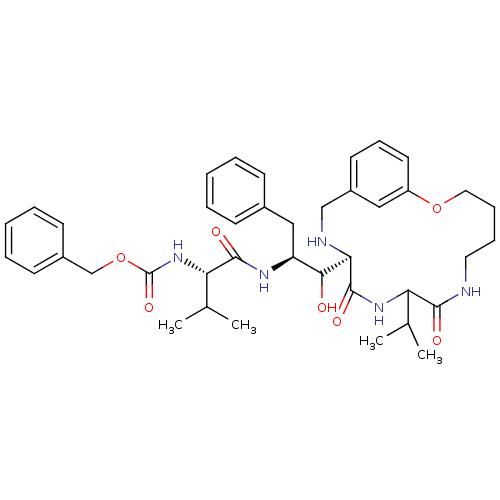 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 28 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.40 | -47.7 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM875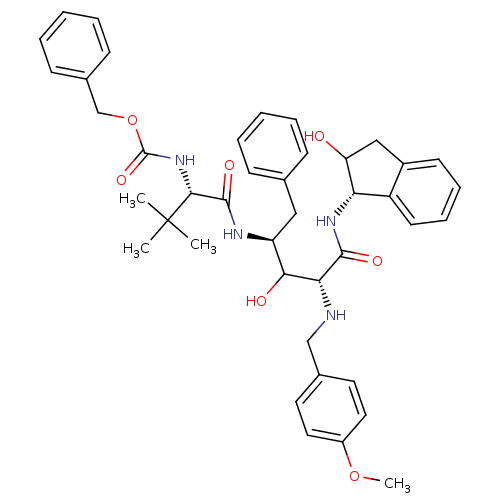 (1OH-2(Cbz-Tle)3PhPr [14]paracyclophane deriv. 2 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.25 | 37 |
Sandoz Research Institute | Assay Description Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ... | J Med Chem 39: 3291-9 (1996) Article DOI: 10.1021/jm950641i BindingDB Entry DOI: 10.7270/Q2P84935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1224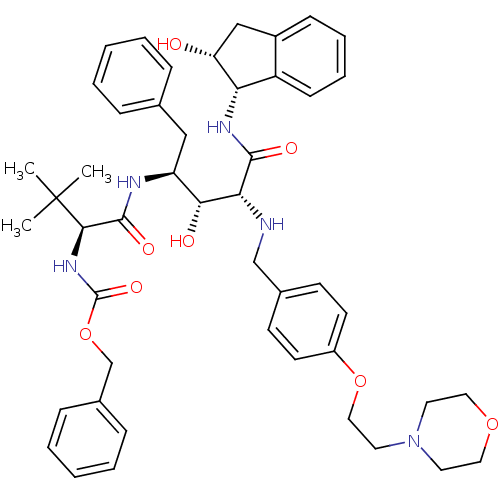 (2-Aminobenzyl-Substituted AHPPA deriv. 42 | benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407634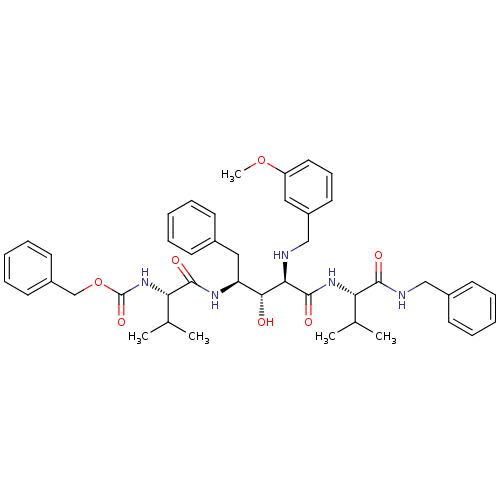 (CHEMBL344972) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407655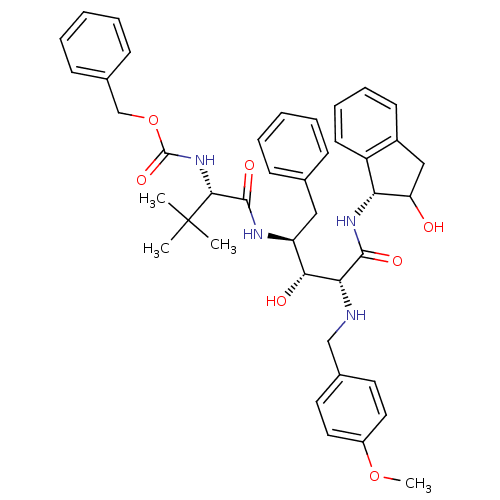 (CHEMBL144259) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407669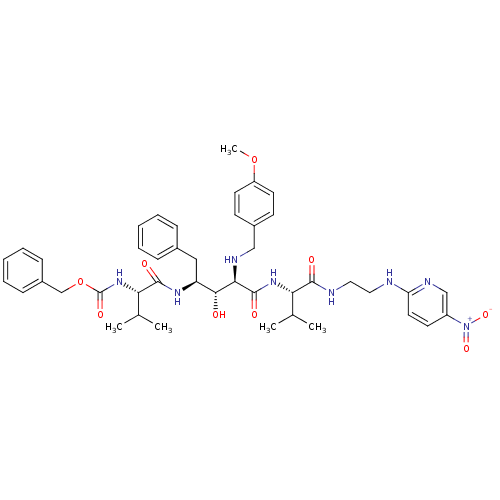 (CHEMBL345003) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407668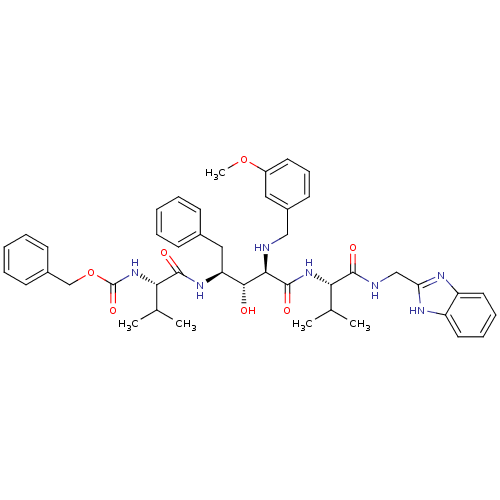 (CHEMBL148062) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM956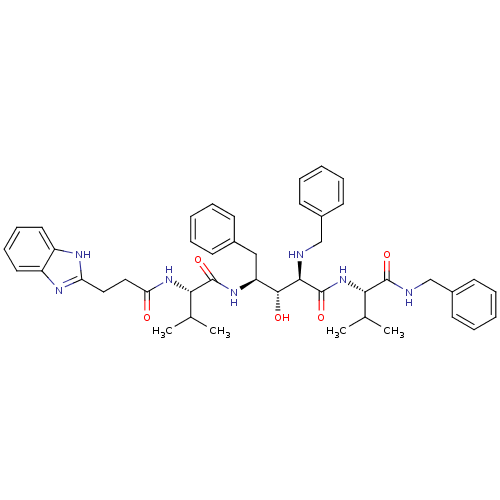 ((2R,3R,4S)-4-[(2S)-2-[3-(1H-1,3-benzodiazol-2-yl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1222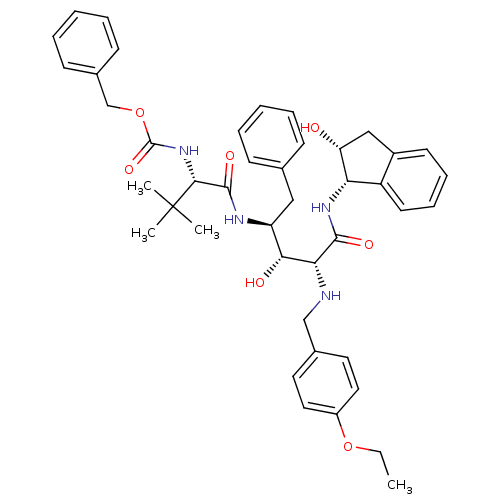 (2-Aminobenzyl-Substituted AHPPA deriv. 40 | benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407657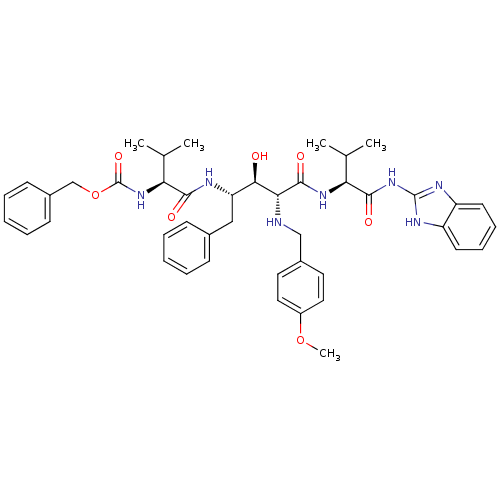 (CHEMBL146363) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SANDOZ Forschungsinstitut Ges. m.b.H Curated by ChEMBL | Assay Description Inhibitory activity was determined against HIV type 1 protease | J Med Chem 38: 4917-28 (1996) BindingDB Entry DOI: 10.7270/Q2FX7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |