 Found 1383 hits with Last Name = 'henry' and Initial = 'r'
Found 1383 hits with Last Name = 'henry' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202094
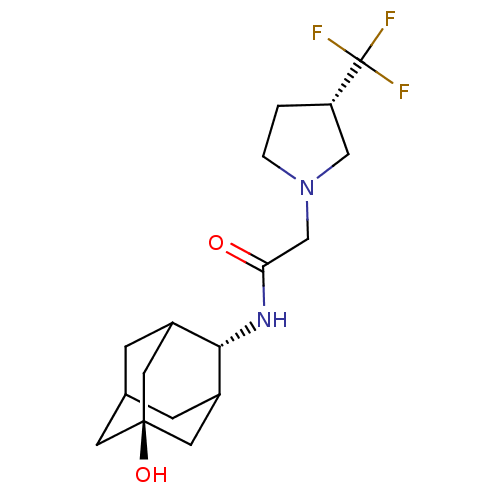
(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50471340

(CHEMBL317912)Show InChI InChI=1S/C13H14N2O/c16-13-6-2-3-9-10(4-1-5-11(9)13)12-7-14-8-15-12/h2-3,6-8,10,16H,1,4-5H2,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards alpha-2B adrenergic receptor of rat neonatal lung in radioligand binding assay |
J Med Chem 47: 3220-35 (2004)
Article DOI: 10.1021/jm030551a
BindingDB Entry DOI: 10.7270/Q2HD7ZD1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202102
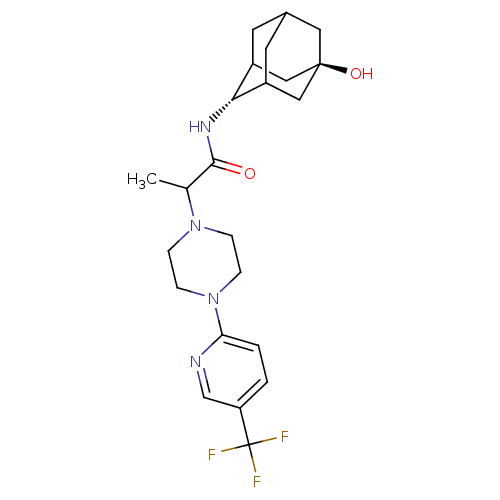
(CHEMBL375156 | N-(5-Hydroxy-adamantan-2-yl)-2-[4-(...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(-1.15,-33.95,;-1.14,-35.49,;.19,-36.26,;.19,-37.8,;1.52,-38.56,;2.85,-37.8,;2.85,-36.25,;1.52,-35.48,;4.19,-38.57,;4.18,-40.11,;5.51,-40.88,;6.84,-40.11,;6.84,-38.56,;5.51,-37.8,;8.18,-40.88,;9.46,-41.72,;8.99,-39.57,;7.38,-42.19,;-2.48,-36.27,;-2.47,-37.81,;-3.81,-35.5,;-5.14,-36.28,;-5.24,-37.8,;-6.33,-39.02,;-7.7,-38.37,;-7.61,-36.79,;-6.5,-35.62,;-7.88,-36.01,;-7.95,-37.5,;-9.42,-37,;-9.22,-38.7,;-6.66,-38.06,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14?,15?,16?,17?,20-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202087
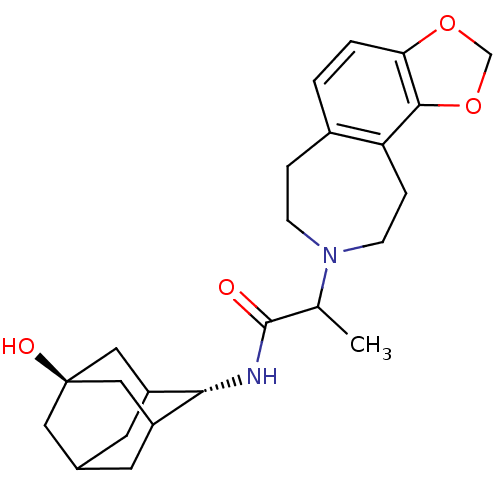
(CHEMBL374728 | N-[(1R,3S)-5-hydroxy-2-adamantyl]-2...)Show SMILES CC(N1CCc2ccc3OCOc3c2CC1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:19.21,wD:26.30,TLB:19:20:28:23.24.25,18:19:28.22.23:25,THB:21:22:25:29.20.19,21:20:28.22.23:25,19:24:28:29.21.20,(.95,-44.73,;.96,-46.27,;2.31,-47.02,;2.21,-48.56,;3.35,-49.6,;4.88,-49.34,;5.66,-50.67,;7.21,-50.65,;7.96,-49.29,;9.45,-48.94,;9.58,-47.41,;8.17,-46.82,;7.17,-47.97,;5.63,-48,;5.05,-46.58,;3.56,-46.15,;-.36,-47.05,;-.34,-48.59,;-1.7,-46.3,;-2.98,-47.15,;-2.99,-48.68,;-3.99,-49.97,;-5.4,-49.41,;-5.41,-47.83,;-4.38,-46.58,;-5.73,-47.07,;-5.71,-48.55,;-7.26,-47.91,;-6.9,-49.84,;-4.38,-49.04,)| Show InChI InChI=1S/C24H32N2O4/c1-14(23(27)25-21-17-8-15-9-18(21)12-24(28,10-15)11-17)26-6-4-16-2-3-20-22(30-13-29-20)19(16)5-7-26/h2-3,14-15,17-18,21,28H,4-13H2,1H3,(H,25,27)/t14?,15?,17?,18?,21-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202085
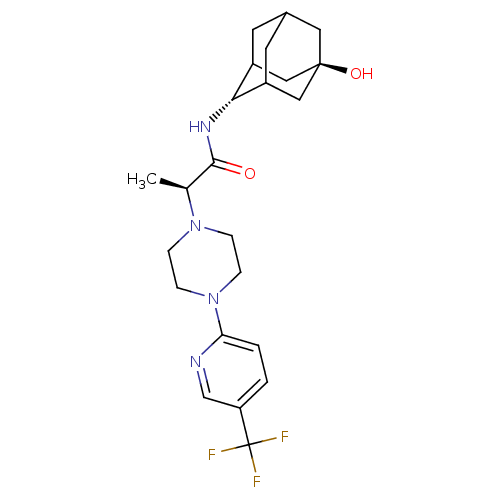
((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202085

((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202087

(CHEMBL374728 | N-[(1R,3S)-5-hydroxy-2-adamantyl]-2...)Show SMILES CC(N1CCc2ccc3OCOc3c2CC1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:19.21,wD:26.30,TLB:19:20:28:23.24.25,18:19:28.22.23:25,THB:21:22:25:29.20.19,21:20:28.22.23:25,19:24:28:29.21.20,(.95,-44.73,;.96,-46.27,;2.31,-47.02,;2.21,-48.56,;3.35,-49.6,;4.88,-49.34,;5.66,-50.67,;7.21,-50.65,;7.96,-49.29,;9.45,-48.94,;9.58,-47.41,;8.17,-46.82,;7.17,-47.97,;5.63,-48,;5.05,-46.58,;3.56,-46.15,;-.36,-47.05,;-.34,-48.59,;-1.7,-46.3,;-2.98,-47.15,;-2.99,-48.68,;-3.99,-49.97,;-5.4,-49.41,;-5.41,-47.83,;-4.38,-46.58,;-5.73,-47.07,;-5.71,-48.55,;-7.26,-47.91,;-6.9,-49.84,;-4.38,-49.04,)| Show InChI InChI=1S/C24H32N2O4/c1-14(23(27)25-21-17-8-15-9-18(21)12-24(28,10-15)11-17)26-6-4-16-2-3-20-22(30-13-29-20)19(16)5-7-26/h2-3,14-15,17-18,21,28H,4-13H2,1H3,(H,25,27)/t14?,15?,17?,18?,21-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202102

(CHEMBL375156 | N-(5-Hydroxy-adamantan-2-yl)-2-[4-(...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(-1.15,-33.95,;-1.14,-35.49,;.19,-36.26,;.19,-37.8,;1.52,-38.56,;2.85,-37.8,;2.85,-36.25,;1.52,-35.48,;4.19,-38.57,;4.18,-40.11,;5.51,-40.88,;6.84,-40.11,;6.84,-38.56,;5.51,-37.8,;8.18,-40.88,;9.46,-41.72,;8.99,-39.57,;7.38,-42.19,;-2.48,-36.27,;-2.47,-37.81,;-3.81,-35.5,;-5.14,-36.28,;-5.24,-37.8,;-6.33,-39.02,;-7.7,-38.37,;-7.61,-36.79,;-6.5,-35.62,;-7.88,-36.01,;-7.95,-37.5,;-9.42,-37,;-9.22,-38.7,;-6.66,-38.06,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14?,15?,16?,17?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202085

((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202093
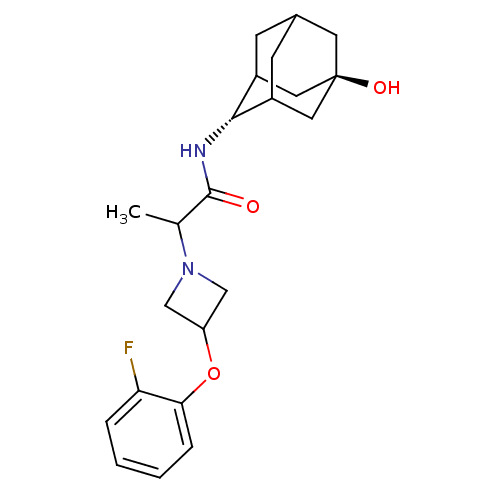
(2-[3-(2-fluoro-phenoxy)-azetidin-1-yl]-N-(5-hydrox...)Show SMILES CC(N1CC(C1)Oc1ccccc1F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:17.18,wD:24.27,TLB:17:18:26:21.22.23,16:17:26.20.21:23,THB:19:20:23:27.18.17,19:18:26.20.21:23,17:22:26:27.19.18,(24.76,-36.3,;24.78,-37.84,;26.12,-38.59,;26.53,-40.07,;28.01,-39.66,;27.6,-38.17,;29.34,-40.41,;30.67,-39.62,;32.01,-40.38,;33.33,-39.6,;33.31,-38.06,;31.96,-37.3,;30.64,-38.09,;29.3,-37.34,;23.45,-38.62,;23.47,-40.16,;22.11,-37.87,;20.83,-38.72,;20.83,-40.25,;19.82,-41.54,;18.42,-40.98,;18.4,-39.39,;19.43,-38.15,;18.09,-38.64,;18.11,-40.12,;16.55,-39.48,;16.92,-41.41,;19.44,-40.61,)| Show InChI InChI=1S/C22H29FN2O3/c1-13(25-11-17(12-25)28-19-5-3-2-4-18(19)23)21(26)24-20-15-6-14-7-16(20)10-22(27,8-14)9-15/h2-5,13-17,20,27H,6-12H2,1H3,(H,24,26)/t13?,14?,15?,16?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202085

((R)-N-[(E)-5-hydroxy-2-adamantyl]-2-{4-[5-(trifluo...)Show SMILES C[C@H](N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,1.0,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(19.54,-43.75,;19.58,-45.29,;20.93,-46.02,;20.92,-47.57,;22.25,-48.34,;23.59,-47.58,;23.59,-46.04,;22.26,-45.26,;24.89,-48.41,;24.81,-49.94,;26.11,-50.77,;27.48,-50.06,;27.54,-48.51,;26.24,-47.69,;28.78,-50.88,;30.09,-51.68,;27.97,-52.19,;29.6,-49.58,;18.27,-46.09,;18.31,-47.63,;16.91,-45.36,;15.6,-46.16,;15.54,-47.69,;14.48,-48.93,;13.09,-48.31,;13.14,-46.73,;14.23,-45.53,;12.86,-45.96,;12.82,-47.45,;11.5,-46.64,;11.58,-48.68,;14.13,-47.98,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14-,15?,16?,17?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202105
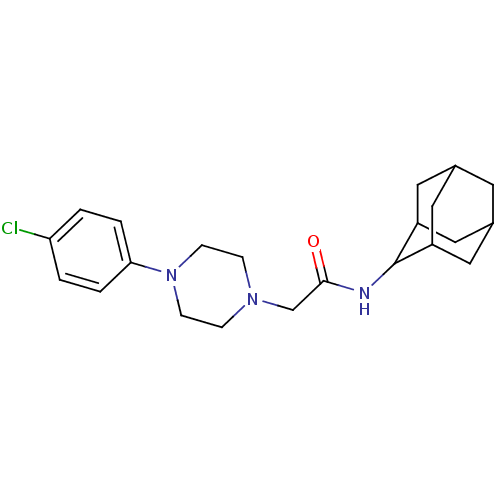
(CHEMBL218093 | N-adamantan-2-yl-2-[4-(4-chloro-phe...)Show SMILES Clc1ccc(cc1)N1CCN(CC(=O)NC2C3CC4CC(C3)CC2C4)CC1 |TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:14:15:19.20.22:24,15:16:19:22.23.24,15:23:19:17.21.16,(23.9,-3.58,;22.58,-2.79,;21.23,-3.53,;19.91,-2.74,;19.94,-1.2,;21.28,-.45,;22.6,-1.24,;18.62,-.4,;17.3,-1.2,;15.96,-.46,;15.93,1.09,;14.56,1.8,;13.26,.96,;13.34,-.58,;11.89,1.66,;10.6,.83,;10.57,-.7,;9.17,-1.03,;7.84,-.53,;6.63,-1.79,;8.14,-1.39,;9.54,-1.97,;8.15,.2,;9.21,1.42,;7.85,.96,;17.24,1.89,;18.59,1.14,)| Show InChI InChI=1S/C22H30ClN3O/c23-19-1-3-20(4-2-19)26-7-5-25(6-8-26)14-21(27)24-22-17-10-15-9-16(12-17)13-18(22)11-15/h1-4,15-18,22H,5-14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202086
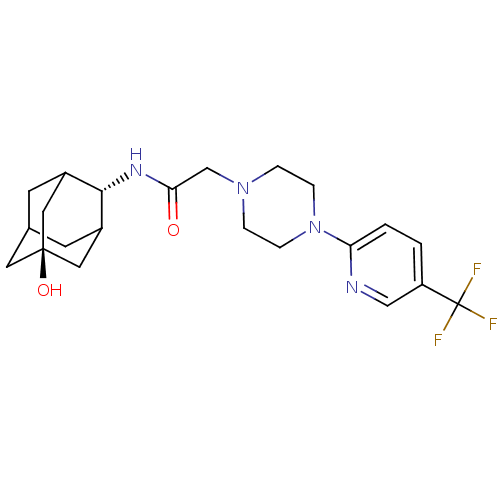
(CHEMBL219142 | N-[(E)-5-hydroxy-2-adamantyl]-2-{4-...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:4:3:30:6.5.7,4:5:2.3.29:30,THB:7:5:2:29.28.30,7:28:2:6.4.5,8:7:2.3.29:30,(1.8,-8.48,;3.15,-9.25,;1.93,-10.52,;3.44,-10.11,;4.84,-10.69,;5.87,-9.43,;4.47,-9.76,;5.9,-7.9,;7.19,-7.07,;8.56,-7.77,;8.64,-9.31,;9.85,-6.93,;11.23,-7.63,;11.25,-9.18,;12.59,-9.92,;13.92,-9.13,;13.88,-7.59,;12.53,-6.84,;15.23,-9.92,;15.2,-11.46,;16.52,-12.26,;17.87,-11.51,;17.89,-9.96,;16.57,-9.18,;19.19,-12.31,;20.51,-13.07,;18.4,-13.63,;19.98,-10.98,;4.51,-7.31,;3.45,-8.53,;3.16,-7.77,)| Show InChI InChI=1S/C22H29F3N4O2/c23-22(24,25)17-1-2-18(26-12-17)29-5-3-28(4-6-29)13-19(30)27-20-15-7-14-8-16(20)11-21(31,9-14)10-15/h1-2,12,14-16,20,31H,3-11,13H2,(H,27,30)/t14?,15?,16?,20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202090
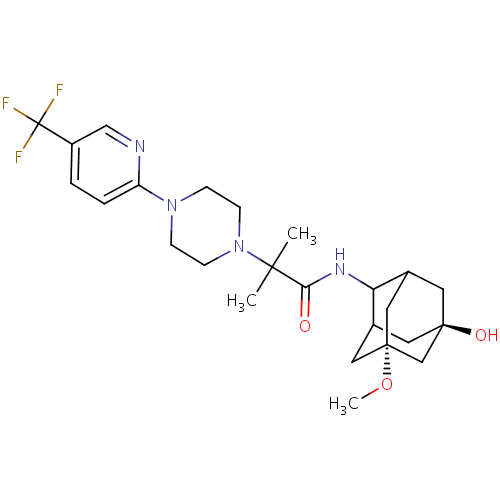
(CHEMBL219443 | N-(7-hydroxy-5-methoxy-adamantan-2-...)Show SMILES CO[C@]12CC3C[C@](O)(CC(C1)C3NC(=O)C(C)(C)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C2 |r,TLB:11:4:34:10.9.8,11:9:34:5.3.4,12:11:34.2.10:8,THB:3:2:8:5.4.11,3:4:34.2.10:8,1:2:8:5.4.11| Show InChI InChI=1S/C25H35F3N4O3/c1-22(2,32-8-6-31(7-9-32)19-5-4-18(14-29-19)25(26,27)28)21(33)30-20-16-10-23(34)11-17(20)13-24(12-16,15-23)35-3/h4-5,14,16-17,20,34H,6-13,15H2,1-3H3,(H,30,33)/t16?,17?,20?,23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202104
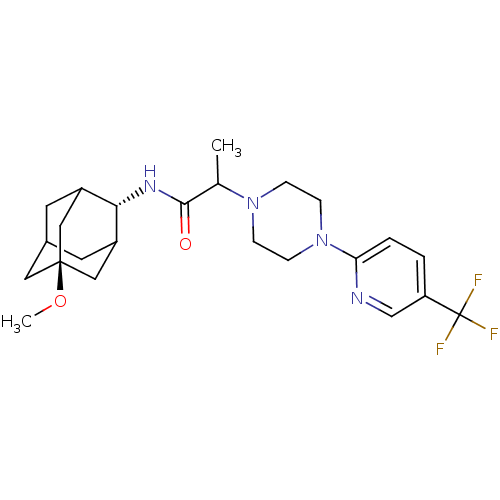
((E)-N-(5-methoxy-adamantan-2-yl)-2-[4-(5-trifluoro...)Show SMILES CO[C@@]12CC3CC(C1)[C@H](NC(=O)C(C)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:8.9,wD:2.1,TLB:5:4:32:7.6.8,5:6:3.4.31:32,THB:8:6:3:31.30.32,8:30:3:7.5.6,9:8:3.4.31:32,(-11.76,-8.87,;-10.48,-7.77,;-8.89,-8.33,;-10.15,-9.55,;-8.63,-9.2,;-7.25,-9.82,;-6.18,-8.59,;-7.59,-8.88,;-6.1,-7.07,;-4.78,-6.27,;-3.43,-7.02,;-3.41,-8.56,;-2.11,-6.23,;-2.14,-4.69,;-.77,-6.98,;-.78,-8.53,;.54,-9.3,;1.88,-8.54,;1.89,-6.99,;.55,-6.21,;3.18,-9.36,;3.11,-10.9,;4.41,-11.72,;5.78,-11.01,;5.84,-9.47,;4.54,-8.65,;7.08,-11.84,;8.38,-12.64,;6.26,-13.14,;7.9,-10.54,;-7.47,-6.43,;-8.56,-7.61,;-8.84,-6.84,)| Show InChI InChI=1S/C24H33F3N4O2/c1-15(22(32)29-21-17-9-16-10-18(21)13-23(11-16,12-17)33-2)30-5-7-31(8-6-30)20-4-3-19(14-28-20)24(25,26)27/h3-4,14-18,21H,5-13H2,1-2H3,(H,29,32)/t15?,16?,17?,18?,21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193321

((Z)-1-(3,5-difluorophenyl)-3-(4-pyridin-2-ylpipera...)Show SMILES CO\N=C(\CCN1CCN(CC1)c1ccccn1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C19H22F2N4O/c1-26-23-18(15-12-16(20)14-17(21)13-15)5-7-24-8-10-25(11-9-24)19-4-2-3-6-22-19/h2-4,6,12-14H,5,7-11H2,1H3/b23-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193331
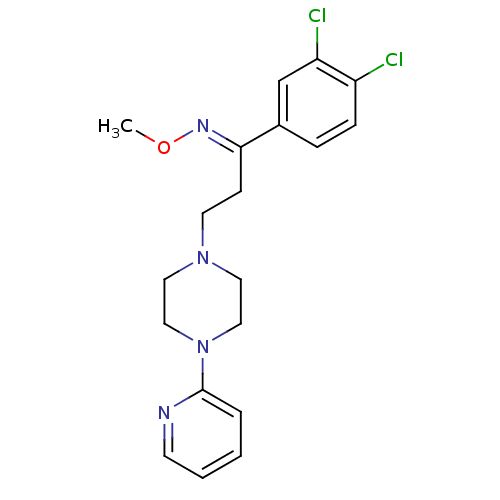
((E)-1-(3,4-dichlorophenyl)-3-(4-pyridin-2-ylpipera...)Show SMILES CO\N=C(/CCN1CCN(CC1)c1ccccn1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H22Cl2N4O/c1-26-23-18(15-5-6-16(20)17(21)14-15)7-9-24-10-12-25(13-11-24)19-4-2-3-8-22-19/h2-6,8,14H,7,9-13H2,1H3/b23-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193296
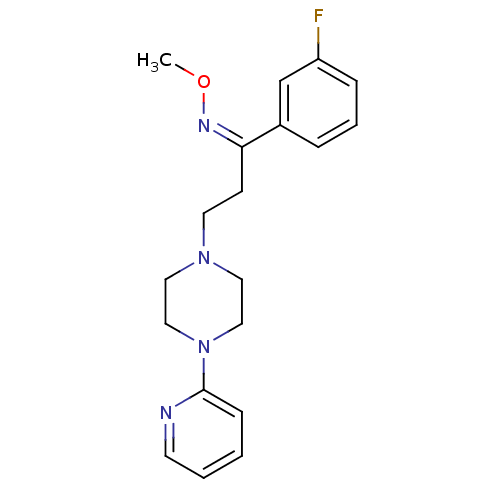
((Z)-1-(3-fluorophenyl)-3-(4-pyridin-2-ylpiperazin-...)Show InChI InChI=1S/C19H23FN4O/c1-25-22-18(16-5-4-6-17(20)15-16)8-10-23-11-13-24(14-12-23)19-7-2-3-9-21-19/h2-7,9,15H,8,10-14H2,1H3/b22-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202093

(2-[3-(2-fluoro-phenoxy)-azetidin-1-yl]-N-(5-hydrox...)Show SMILES CC(N1CC(C1)Oc1ccccc1F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:17.18,wD:24.27,TLB:17:18:26:21.22.23,16:17:26.20.21:23,THB:19:20:23:27.18.17,19:18:26.20.21:23,17:22:26:27.19.18,(24.76,-36.3,;24.78,-37.84,;26.12,-38.59,;26.53,-40.07,;28.01,-39.66,;27.6,-38.17,;29.34,-40.41,;30.67,-39.62,;32.01,-40.38,;33.33,-39.6,;33.31,-38.06,;31.96,-37.3,;30.64,-38.09,;29.3,-37.34,;23.45,-38.62,;23.47,-40.16,;22.11,-37.87,;20.83,-38.72,;20.83,-40.25,;19.82,-41.54,;18.42,-40.98,;18.4,-39.39,;19.43,-38.15,;18.09,-38.64,;18.11,-40.12,;16.55,-39.48,;16.92,-41.41,;19.44,-40.61,)| Show InChI InChI=1S/C22H29FN2O3/c1-13(25-11-17(12-25)28-19-5-3-2-4-18(19)23)21(26)24-20-15-6-14-7-16(20)10-22(27,8-14)9-15/h2-5,13-17,20,27H,6-12H2,1H3,(H,24,26)/t13?,14?,15?,16?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202097
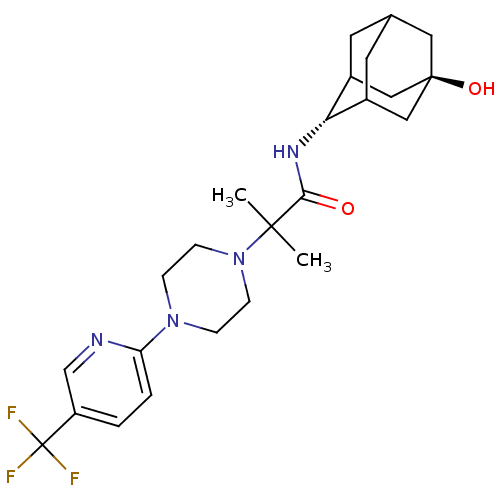
(CHEMBL424937 | N-[(E)-5-hydroxy-2-adamantyl]-2-met...)Show SMILES CC(C)(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.23,wD:29.32,TLB:22:23:31:26.27.28,21:22:31.25.26:28,THB:24:25:28:32.23.22,24:23:31.25.26:28,22:27:31:32.24.23,(20.61,-48.3,;19.75,-47.03,;18.89,-45.75,;21.02,-46.15,;22.39,-46.86,;23.68,-46.03,;23.61,-44.49,;22.24,-43.79,;20.94,-44.62,;24.94,-43.71,;26.27,-44.47,;27.6,-43.69,;27.58,-42.15,;26.23,-41.39,;24.91,-42.18,;28.91,-41.36,;30.21,-40.56,;29.7,-42.68,;28.12,-40.04,;18.46,-47.89,;18.49,-49.43,;17.12,-47.14,;15.8,-47.93,;15.72,-49.46,;14.65,-50.69,;13.27,-50.06,;13.33,-48.48,;14.43,-47.29,;13.06,-47.71,;13,-49.19,;11.69,-48.37,;11.75,-50.41,;14.31,-49.74,)| Show InChI InChI=1S/C24H33F3N4O2/c1-22(2,21(32)29-20-16-9-15-10-17(20)13-23(33,11-15)12-16)31-7-5-30(6-8-31)19-4-3-18(14-28-19)24(25,26)27/h3-4,14-17,20,33H,5-13H2,1-2H3,(H,29,32)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202086

(CHEMBL219142 | N-[(E)-5-hydroxy-2-adamantyl]-2-{4-...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:4:3:30:6.5.7,4:5:2.3.29:30,THB:7:5:2:29.28.30,7:28:2:6.4.5,8:7:2.3.29:30,(1.8,-8.48,;3.15,-9.25,;1.93,-10.52,;3.44,-10.11,;4.84,-10.69,;5.87,-9.43,;4.47,-9.76,;5.9,-7.9,;7.19,-7.07,;8.56,-7.77,;8.64,-9.31,;9.85,-6.93,;11.23,-7.63,;11.25,-9.18,;12.59,-9.92,;13.92,-9.13,;13.88,-7.59,;12.53,-6.84,;15.23,-9.92,;15.2,-11.46,;16.52,-12.26,;17.87,-11.51,;17.89,-9.96,;16.57,-9.18,;19.19,-12.31,;20.51,-13.07,;18.4,-13.63,;19.98,-10.98,;4.51,-7.31,;3.45,-8.53,;3.16,-7.77,)| Show InChI InChI=1S/C22H29F3N4O2/c23-22(24,25)17-1-2-18(26-12-17)29-5-3-28(4-6-29)13-19(30)27-20-15-7-14-8-16(20)11-21(31,9-14)10-15/h1-2,12,14-16,20,31H,3-11,13H2,(H,27,30)/t14?,15?,16?,20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193330
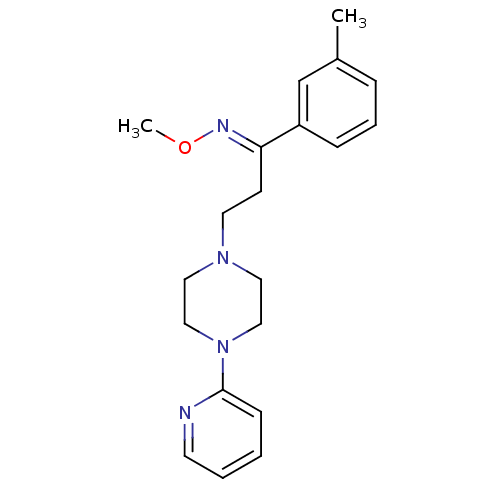
((E)-3-(4-pyridin-2-ylpiperazin-1-yl)-1-(m-tolyl)pr...)Show InChI InChI=1S/C20H26N4O/c1-17-6-5-7-18(16-17)19(22-25-2)9-11-23-12-14-24(15-13-23)20-8-3-4-10-21-20/h3-8,10,16H,9,11-15H2,1-2H3/b22-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202097

(CHEMBL424937 | N-[(E)-5-hydroxy-2-adamantyl]-2-met...)Show SMILES CC(C)(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:22.23,wD:29.32,TLB:22:23:31:26.27.28,21:22:31.25.26:28,THB:24:25:28:32.23.22,24:23:31.25.26:28,22:27:31:32.24.23,(20.61,-48.3,;19.75,-47.03,;18.89,-45.75,;21.02,-46.15,;22.39,-46.86,;23.68,-46.03,;23.61,-44.49,;22.24,-43.79,;20.94,-44.62,;24.94,-43.71,;26.27,-44.47,;27.6,-43.69,;27.58,-42.15,;26.23,-41.39,;24.91,-42.18,;28.91,-41.36,;30.21,-40.56,;29.7,-42.68,;28.12,-40.04,;18.46,-47.89,;18.49,-49.43,;17.12,-47.14,;15.8,-47.93,;15.72,-49.46,;14.65,-50.69,;13.27,-50.06,;13.33,-48.48,;14.43,-47.29,;13.06,-47.71,;13,-49.19,;11.69,-48.37,;11.75,-50.41,;14.31,-49.74,)| Show InChI InChI=1S/C24H33F3N4O2/c1-22(2,21(32)29-20-16-9-15-10-17(20)13-23(33,11-15)12-16)31-7-5-30(6-8-31)19-4-3-18(14-28-19)24(25,26)27/h3-4,14-17,20,33H,5-13H2,1-2H3,(H,29,32)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50471340

(CHEMBL317912)Show InChI InChI=1S/C13H14N2O/c16-13-6-2-3-9-10(4-1-5-11(9)13)12-7-14-8-15-12/h2-3,6-8,10,16H,1,4-5H2,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards alpha-2A adrenergic receptor of human clone in radioligand binding assay |
J Med Chem 47: 3220-35 (2004)
Article DOI: 10.1021/jm030551a
BindingDB Entry DOI: 10.7270/Q2HD7ZD1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202095

(CHEMBL415429 | E-(2R)-(3R-fluoro-pyrrolidin-1-yl)-...)Show SMILES C[C@@H](N1CC[C@@H](F)C1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,TLB:11:12:20:15.16.17,10:11:20.14.15:17,THB:13:14:17:21.12.11,13:12:20.14.15:17,11:16:20:21.13.12| Show InChI InChI=1S/C17H27FN2O2/c1-10(20-3-2-14(18)9-20)16(21)19-15-12-4-11-5-13(15)8-17(22,6-11)7-12/h10-15,22H,2-9H2,1H3,(H,19,21)/t10-,11?,12?,13?,14-,15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202103
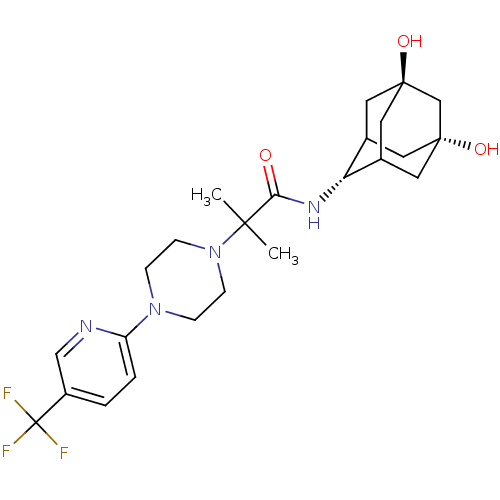
(CHEMBL374922 | N-(5,7-dihydroxy-adamantan-2-yl)-2-...)Show SMILES CC(C)(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2C[C@]3(O)CC1C[C@](O)(C2)C3 |r,wU:22.23,30.33,wD:25.27,TLB:32:30:27:24.23.22,22:23:33:29.28.27,22:28:33:24.32.23,21:22:33.30.29:27,31:30:27:24.23.22,THB:32:23:33.30.29:27,(-1.75,-43.94,;-2.61,-42.66,;-3.47,-41.38,;-1.34,-41.79,;.03,-42.5,;1.32,-41.67,;1.25,-40.13,;-.12,-39.43,;-1.42,-40.26,;2.57,-39.35,;3.91,-40.11,;5.24,-39.32,;5.22,-37.78,;3.87,-37.03,;2.55,-37.81,;6.55,-37,;7.85,-36.2,;7.34,-38.32,;5.76,-35.67,;-3.9,-43.52,;-3.87,-45.06,;-5.25,-42.77,;-6.57,-43.57,;-6.65,-45.09,;-8.06,-45.38,;-9.36,-44.83,;-10.68,-44.01,;-9.3,-43.34,;-7.93,-42.93,;-9.03,-44.11,;-9.09,-45.7,;-9.16,-47.23,;-7.72,-46.32,;-10.61,-46.05,)| Show InChI InChI=1S/C24H33F3N4O3/c1-21(2,20(32)29-19-15-9-22(33)11-16(19)12-23(34,10-15)14-22)31-7-5-30(6-8-31)18-4-3-17(13-28-18)24(25,26)27/h3-4,13,15-16,19,33-34H,5-12,14H2,1-2H3,(H,29,32)/t15?,16?,19-,22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193290
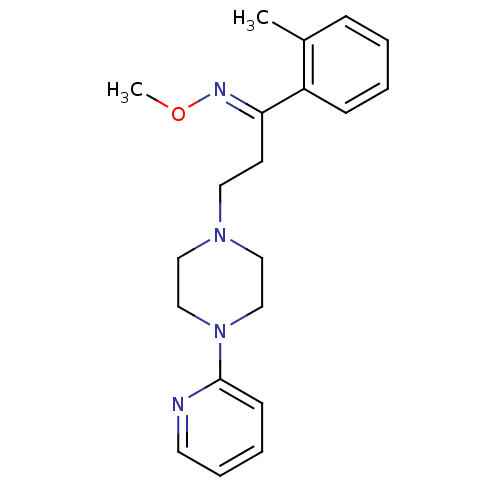
((E)-3-(4-pyridin-2-ylpiperazin-1-yl)-1-(o-tolyl)pr...)Show InChI InChI=1S/C20H26N4O/c1-17-7-3-4-8-18(17)19(22-25-2)10-12-23-13-15-24(16-14-23)20-9-5-6-11-21-20/h3-9,11H,10,12-16H2,1-2H3/b22-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193361
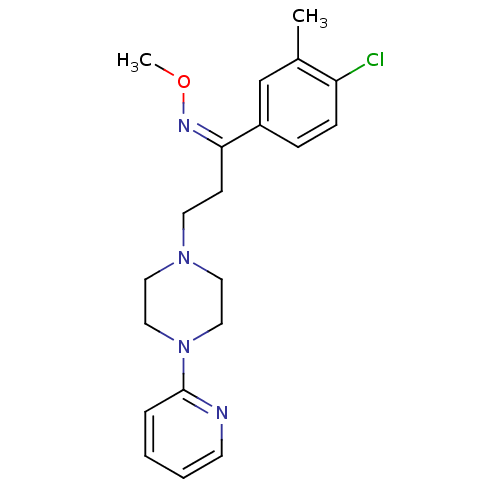
((Z)-1-(4-chloro-3-methylphenyl)-3-(4-pyridin-2-ylp...)Show SMILES CO\N=C(\CCN1CCN(CC1)c1ccccn1)c1ccc(Cl)c(C)c1 Show InChI InChI=1S/C20H25ClN4O/c1-16-15-17(6-7-18(16)21)19(23-26-2)8-10-24-11-13-25(14-12-24)20-5-3-4-9-22-20/h3-7,9,15H,8,10-14H2,1-2H3/b23-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50118703
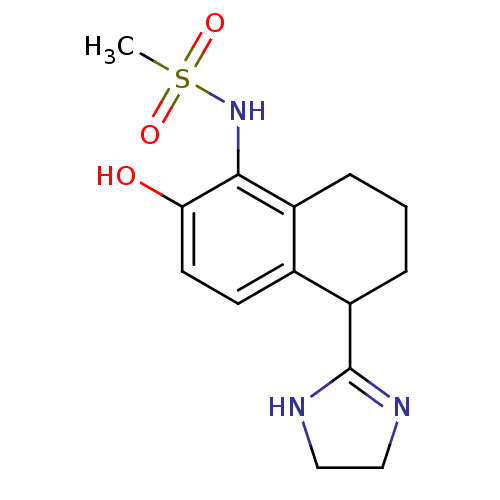
(CHEMBL109783 | N-[5-(4,5-Dihydro-1H-imidazol-2-yl)...)Show SMILES CS(=O)(=O)Nc1c(O)ccc2C(CCCc12)C1=NCCN1 |t:18| Show InChI InChI=1S/C14H19N3O3S/c1-21(19,20)17-13-10-3-2-4-11(14-15-7-8-16-14)9(10)5-6-12(13)18/h5-6,11,17-18H,2-4,7-8H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards alpha-1A adrenergic receptor of rat submaxillary gland in radioligand binding assay |
J Med Chem 47: 3220-35 (2004)
Article DOI: 10.1021/jm030551a
BindingDB Entry DOI: 10.7270/Q2HD7ZD1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080367

((R)-1-{2-[(Z)-2-(2-Chloro-phenyl)-2-phenyl-vinylox...)Show SMILES OC(=O)[C@@H]1CCCN(CCO\C=C(\c2ccccc2)c2ccccc2Cl)C1 Show InChI InChI=1S/C22H24ClNO3/c23-21-11-5-4-10-19(21)20(17-7-2-1-3-8-17)16-27-14-13-24-12-6-9-18(15-24)22(25)26/h1-5,7-8,10-11,16,18H,6,9,12-15H2,(H,25,26)/b20-16-/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080358

(1-{2-[2,2-Bis-(2-chloro-phenyl)-vinyloxy]-ethyl}-1...)Show SMILES [#8]-[#6](=O)-[#6]-1=[#6]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]\[#6]=[#6](\c2ccccc2Cl)-c2ccccc2Cl)-[#6]-1 |t:3| Show InChI InChI=1S/C22H21Cl2NO3/c23-20-9-3-1-7-17(20)19(18-8-2-4-10-21(18)24)15-28-13-12-25-11-5-6-16(14-25)22(26)27/h1-4,6-10,15H,5,11-14H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202095

(CHEMBL415429 | E-(2R)-(3R-fluoro-pyrrolidin-1-yl)-...)Show SMILES C[C@@H](N1CC[C@@H](F)C1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,TLB:11:12:20:15.16.17,10:11:20.14.15:17,THB:13:14:17:21.12.11,13:12:20.14.15:17,11:16:20:21.13.12| Show InChI InChI=1S/C17H27FN2O2/c1-10(20-3-2-14(18)9-20)16(21)19-15-12-4-11-5-13(15)8-17(22,6-11)7-12/h10-15,22H,2-9H2,1H3,(H,19,21)/t10-,11?,12?,13?,14-,15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202100
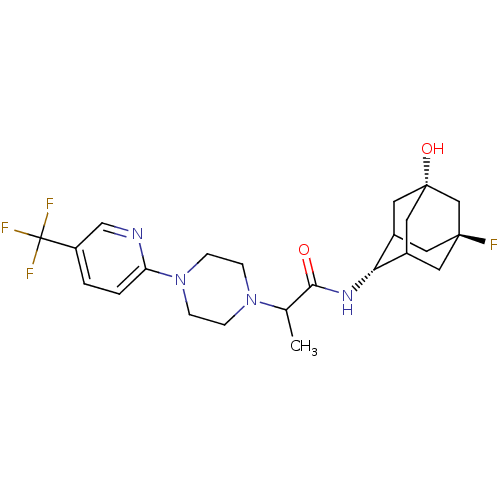
(CHEMBL218197 | N-(5-fluoro-7-hydroxyadamantan-2-yl...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2C[C@@]3(O)CC1C[C@@](F)(C2)C3 |r,wU:21.22,24.26,wD:29.32,TLB:21:22:32:26.27.28,21:27:32:31.23.22,20:21:32.24.26:28,THB:23:24:28:31.22.21,23:22:32.24.26:28,25:24:28:31.22.21,(19.28,-5.83,;19.3,-7.37,;20.64,-8.12,;20.63,-9.67,;21.96,-10.44,;23.3,-9.68,;23.3,-8.14,;21.97,-7.36,;24.6,-10.51,;24.52,-12.04,;25.82,-12.87,;27.19,-12.16,;27.25,-10.61,;25.95,-9.79,;28.49,-12.98,;29.79,-13.78,;27.67,-14.29,;29.31,-11.68,;17.98,-8.16,;18.01,-9.7,;16.63,-7.42,;15.31,-8.21,;15.23,-9.74,;14.16,-10.97,;12.79,-10.34,;12.72,-11.88,;12.85,-8.76,;13.95,-7.57,;12.58,-7.99,;12.52,-9.47,;11.16,-8.87,;13.82,-10.02,;11.27,-10.69,)| Show InChI InChI=1S/C23H30F4N4O2/c1-14(20(32)29-19-15-8-21(24)9-16(19)11-22(33,10-15)13-21)30-4-6-31(7-5-30)18-3-2-17(12-28-18)23(25,26)27/h2-3,12,14-16,19,33H,4-11,13H2,1H3,(H,29,32)/t14?,15?,16?,19-,21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080382
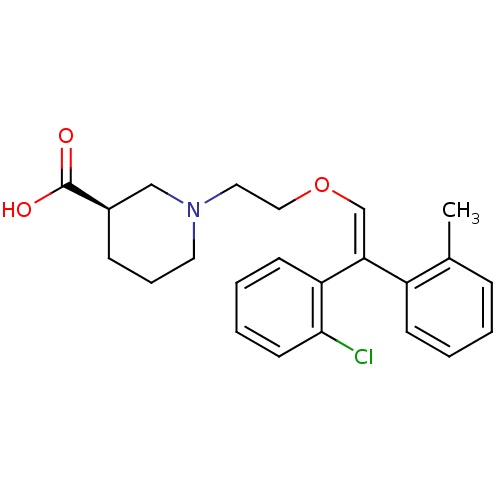
((R)-1-{2-[(Z)-2-(2-Chloro-phenyl)-2-o-tolyl-vinylo...)Show SMILES Cc1ccccc1\C(=C\OCCN1CCC[C@H](C1)C(O)=O)c1ccccc1Cl Show InChI InChI=1S/C23H26ClNO3/c1-17-7-2-3-9-19(17)21(20-10-4-5-11-22(20)24)16-28-14-13-25-12-6-8-18(15-25)23(26)27/h2-5,7,9-11,16,18H,6,8,12-15H2,1H3,(H,26,27)/b21-16-/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202087

(CHEMBL374728 | N-[(1R,3S)-5-hydroxy-2-adamantyl]-2...)Show SMILES CC(N1CCc2ccc3OCOc3c2CC1)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:19.21,wD:26.30,TLB:19:20:28:23.24.25,18:19:28.22.23:25,THB:21:22:25:29.20.19,21:20:28.22.23:25,19:24:28:29.21.20,(.95,-44.73,;.96,-46.27,;2.31,-47.02,;2.21,-48.56,;3.35,-49.6,;4.88,-49.34,;5.66,-50.67,;7.21,-50.65,;7.96,-49.29,;9.45,-48.94,;9.58,-47.41,;8.17,-46.82,;7.17,-47.97,;5.63,-48,;5.05,-46.58,;3.56,-46.15,;-.36,-47.05,;-.34,-48.59,;-1.7,-46.3,;-2.98,-47.15,;-2.99,-48.68,;-3.99,-49.97,;-5.4,-49.41,;-5.41,-47.83,;-4.38,-46.58,;-5.73,-47.07,;-5.71,-48.55,;-7.26,-47.91,;-6.9,-49.84,;-4.38,-49.04,)| Show InChI InChI=1S/C24H32N2O4/c1-14(23(27)25-21-17-8-15-9-18(21)12-24(28,10-15)11-17)26-6-4-16-2-3-20-22(30-13-29-20)19(16)5-7-26/h2-3,14-15,17-18,21,28H,4-13H2,1H3,(H,25,27)/t14?,15?,17?,18?,21-,24- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193307
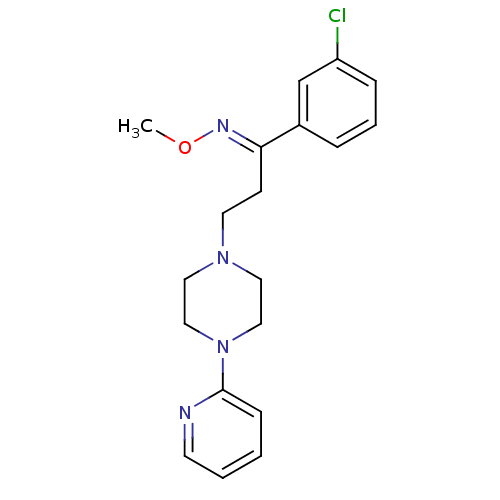
((E)-1-(3-chlorophenyl)-3-(4-pyridin-2-ylpiperazin-...)Show InChI InChI=1S/C19H23ClN4O/c1-25-22-18(16-5-4-6-17(20)15-16)8-10-23-11-13-24(14-12-23)19-7-2-3-9-21-19/h2-7,9,15H,8,10-14H2,1H3/b22-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193350
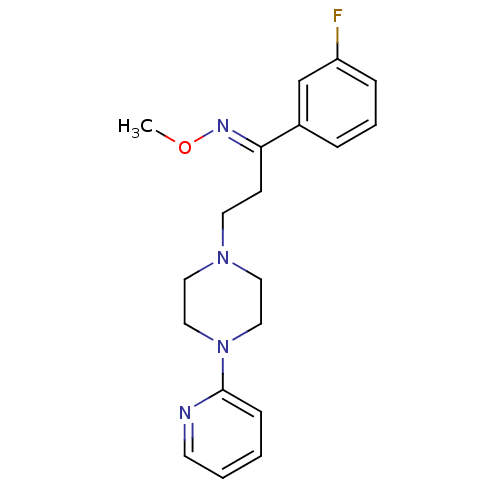
((E)-1-(3-fluorophenyl)-3-(4-pyridin-2-ylpiperazin-...)Show InChI InChI=1S/C19H23FN4O/c1-25-22-18(16-5-4-6-17(20)15-16)8-10-23-11-13-24(14-12-23)19-7-2-3-9-21-19/h2-7,9,15H,8,10-14H2,1H3/b22-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
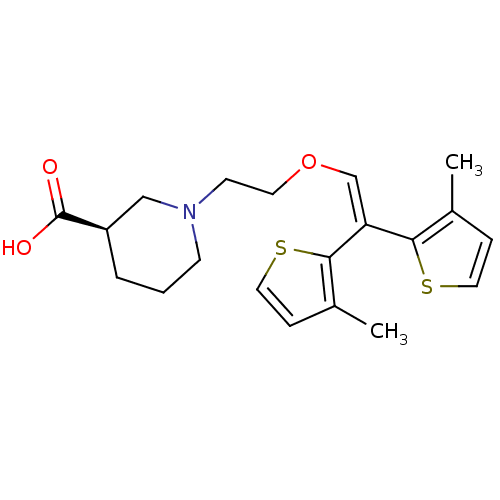
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080337
((R)-1-{2-[2,2-Bis-(3-methyl-thiophen-2-yl)-vinylox...)Show SMILES [#6]-c1ccsc1\[#6](=[#6]/[#8]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6@H](-[#6]-1)-[#6](-[#8])=O)-c1sccc1-[#6] Show InChI InChI=1S/C20H25NO3S2/c1-14-5-10-25-18(14)17(19-15(2)6-11-26-19)13-24-9-8-21-7-3-4-16(12-21)20(22)23/h5-6,10-11,13,16H,3-4,7-9,12H2,1-2H3,(H,22,23)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202091
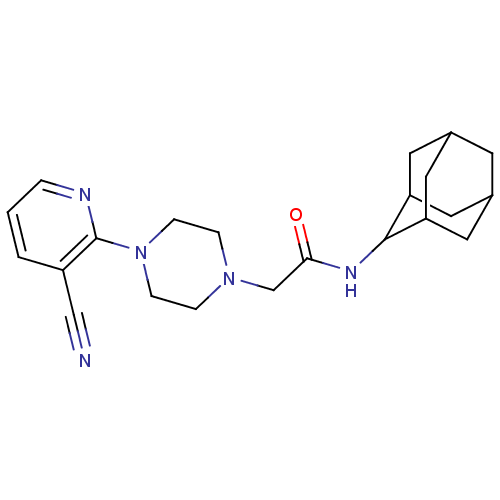
(CHEMBL384364 | N-adamantan-2-yl-2-[4-(3-cyano-pyri...)Show SMILES O=C(CN1CCN(CC1)c1ncccc1C#N)NC1C2CC3CC(C2)CC1C3 |TLB:24:23:27:20.19.18,24:19:22.23.25:27,THB:18:19:22:25.26.27,18:26:22:20.24.19,17:18:22.23.25:27,(-3.45,-1.05,;-3.53,.49,;-2.23,1.33,;-.86,.63,;-.83,-.92,;.51,-1.66,;1.83,-.87,;1.8,.67,;.45,1.42,;3.15,-1.66,;4.49,-.92,;5.8,-1.7,;5.78,-3.25,;4.43,-4,;3.11,-3.2,;1.76,-3.94,;.42,-4.69,;-4.9,1.19,;-6.19,.36,;-6.22,-1.17,;-7.62,-1.5,;-8.94,-.99,;-10.15,-2.26,;-8.65,-1.85,;-7.25,-2.43,;-8.63,-.27,;-7.58,.95,;-8.93,.49,)| Show InChI InChI=1S/C22H29N5O/c23-13-17-2-1-3-24-22(17)27-6-4-26(5-7-27)14-20(28)25-21-18-9-15-8-16(11-18)12-19(21)10-15/h1-3,15-16,18-19,21H,4-12,14H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
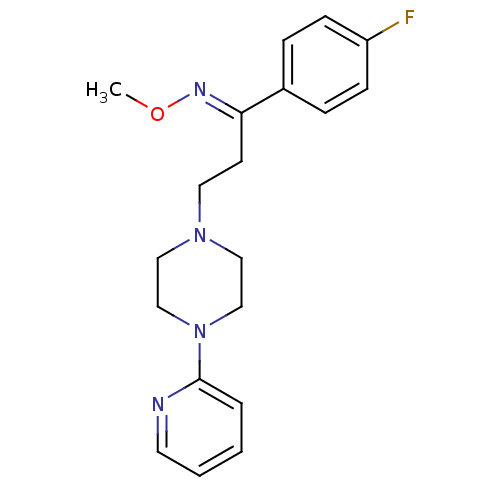
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193378
((E)-1-(4-fluorophenyl)-3-(4-pyridin-2-ylpiperazin-...)Show InChI InChI=1S/C19H23FN4O/c1-25-22-18(16-5-7-17(20)8-6-16)9-11-23-12-14-24(15-13-23)19-4-2-3-10-21-19/h2-8,10H,9,11-15H2,1H3/b22-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202102

(CHEMBL375156 | N-(5-Hydroxy-adamantan-2-yl)-2-[4-(...)Show SMILES CC(N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:21:22:30:25.26.27,20:21:30.24.25:27,THB:23:24:27:31.22.21,23:22:30.24.25:27,21:26:30:31.23.22,(-1.15,-33.95,;-1.14,-35.49,;.19,-36.26,;.19,-37.8,;1.52,-38.56,;2.85,-37.8,;2.85,-36.25,;1.52,-35.48,;4.19,-38.57,;4.18,-40.11,;5.51,-40.88,;6.84,-40.11,;6.84,-38.56,;5.51,-37.8,;8.18,-40.88,;9.46,-41.72,;8.99,-39.57,;7.38,-42.19,;-2.48,-36.27,;-2.47,-37.81,;-3.81,-35.5,;-5.14,-36.28,;-5.24,-37.8,;-6.33,-39.02,;-7.7,-38.37,;-7.61,-36.79,;-6.5,-35.62,;-7.88,-36.01,;-7.95,-37.5,;-9.42,-37,;-9.22,-38.7,;-6.66,-38.06,)| Show InChI InChI=1S/C23H31F3N4O2/c1-14(21(31)28-20-16-8-15-9-17(20)12-22(32,10-15)11-16)29-4-6-30(7-5-29)19-3-2-18(13-27-19)23(24,25)26/h2-3,13-17,20,32H,4-12H2,1H3,(H,28,31)/t14?,15?,16?,17?,20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080376

((R)-1-{2-[(E)-2-(3-Methyl-thiophen-2-yl)-2-o-tolyl...)Show SMILES Cc1ccsc1\C(=C\OCCN1CCC[C@H](C1)C(O)=O)c1ccccc1C Show InChI InChI=1S/C22H27NO3S/c1-16-6-3-4-8-19(16)20(21-17(2)9-13-27-21)15-26-12-11-23-10-5-7-18(14-23)22(24)25/h3-4,6,8-9,13,15,18H,5,7,10-12,14H2,1-2H3,(H,24,25)/b20-15+/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202104

((E)-N-(5-methoxy-adamantan-2-yl)-2-[4-(5-trifluoro...)Show SMILES CO[C@@]12CC3CC(C1)[C@H](NC(=O)C(C)N1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:8.9,wD:2.1,TLB:5:4:32:7.6.8,5:6:3.4.31:32,THB:8:6:3:31.30.32,8:30:3:7.5.6,9:8:3.4.31:32,(-11.76,-8.87,;-10.48,-7.77,;-8.89,-8.33,;-10.15,-9.55,;-8.63,-9.2,;-7.25,-9.82,;-6.18,-8.59,;-7.59,-8.88,;-6.1,-7.07,;-4.78,-6.27,;-3.43,-7.02,;-3.41,-8.56,;-2.11,-6.23,;-2.14,-4.69,;-.77,-6.98,;-.78,-8.53,;.54,-9.3,;1.88,-8.54,;1.89,-6.99,;.55,-6.21,;3.18,-9.36,;3.11,-10.9,;4.41,-11.72,;5.78,-11.01,;5.84,-9.47,;4.54,-8.65,;7.08,-11.84,;8.38,-12.64,;6.26,-13.14,;7.9,-10.54,;-7.47,-6.43,;-8.56,-7.61,;-8.84,-6.84,)| Show InChI InChI=1S/C24H33F3N4O2/c1-15(22(32)29-21-17-9-16-10-18(21)13-23(11-16,12-17)33-2)30-5-7-31(8-6-30)20-4-3-19(14-28-20)24(25,26)27/h3-4,14-18,21H,5-13H2,1-2H3,(H,29,32)/t15?,16?,17?,18?,21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080327

(1-{2-[(Z)-2-(2-Fluoro-phenyl)-2-o-tolyl-vinyloxy]-...)Show SMILES Cc1ccccc1\C(=C\OCCN1CCC=C(C1)C(O)=O)c1ccccc1F |c:16| Show InChI InChI=1S/C23H24FNO3/c1-17-7-2-3-9-19(17)21(20-10-4-5-11-22(20)24)16-28-14-13-25-12-6-8-18(15-25)23(26)27/h2-5,7-11,16H,6,12-15H2,1H3,(H,26,27)/b21-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080335

((R)-1-[2-(2,2-Di-o-tolyl-vinyloxy)-ethyl]-piperidi...)Show SMILES [#6]-c1ccccc1\[#6](=[#6]/[#8]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6@H](-[#6]-1)-[#6](-[#8])=O)-c1ccccc1-[#6] Show InChI InChI=1S/C24H29NO3/c1-18-8-3-5-11-21(18)23(22-12-6-4-9-19(22)2)17-28-15-14-25-13-7-10-20(16-25)24(26)27/h3-6,8-9,11-12,17,20H,7,10,13-16H2,1-2H3,(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50193311
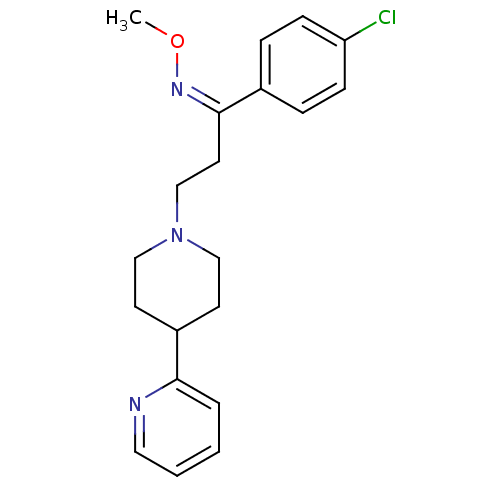
((Z)-1-(4-chlorophenyl)-3-(3',4',5',6'-tetrahydro-2...)Show InChI InChI=1S/C20H24ClN3O/c1-25-23-20(16-5-7-18(21)8-6-16)11-15-24-13-9-17(10-14-24)19-4-2-3-12-22-19/h2-8,12,17H,9-11,13-15H2,1H3/b23-20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]PIPAT from human recombinant D2L receptor expressed in HEK293 cells |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50193357
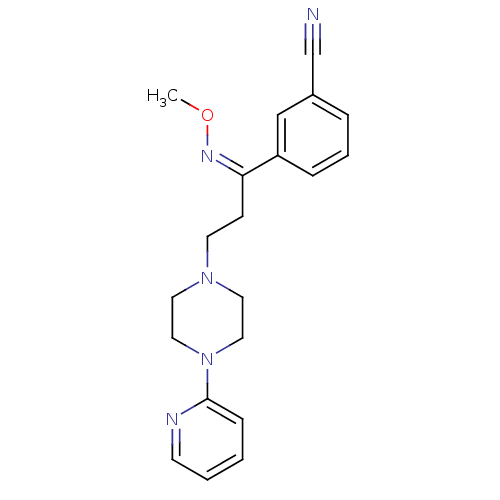
((Z)-4-[1-methoxyimino-3-(4-pyridin-2-ylpiperazin-1...)Show InChI InChI=1S/C20H23N5O/c1-26-23-19(18-6-4-5-17(15-18)16-21)8-10-24-11-13-25(14-12-24)20-7-2-3-9-22-20/h2-7,9,15H,8,10-14H2,1H3/b23-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]A-369508 from human recombinant D4.4 receptor expressed in HEK293 cell membrane |
J Med Chem 49: 5093-109 (2006)
Article DOI: 10.1021/jm060279f
BindingDB Entry DOI: 10.7270/Q2CJ8D4B |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50080375

((R)-1-[2-((E)-2-Phenyl-2-o-tolyl-vinyloxy)-ethyl]-...)Show SMILES Cc1ccccc1\C(=C\OCCN1CCC[C@H](C1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H27NO3/c1-18-8-5-6-12-21(18)22(19-9-3-2-4-10-19)17-27-15-14-24-13-7-11-20(16-24)23(25)26/h2-6,8-10,12,17,20H,7,11,13-16H2,1H3,(H,25,26)/b22-17+/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]GABA uptake at the Sodium- and chloride-dependent GABA transporter 1 (GAT-1) uptake site in rat brain synaptosomes |
J Med Chem 42: 3447-62 (1999)
Article DOI: 10.1021/jm981027k
BindingDB Entry DOI: 10.7270/Q2057GMV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data