

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50266003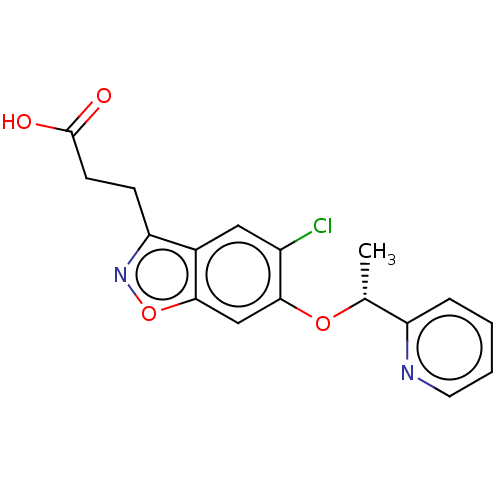 (CHEMBL4091152) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate ... | J Med Chem 60: 3383-3404 (2017) Article DOI: 10.1021/acs.jmedchem.7b00055 BindingDB Entry DOI: 10.7270/Q26112SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50266041 (CHEMBL4070212) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... | J Med Chem 60: 3383-3404 (2017) Article DOI: 10.1021/acs.jmedchem.7b00055 BindingDB Entry DOI: 10.7270/Q26112SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418268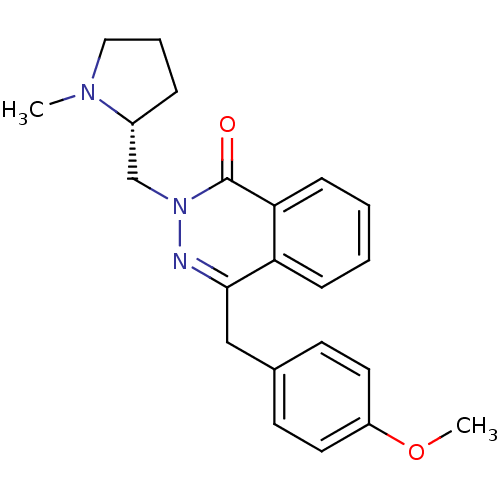 (CHEMBL1767137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391708 (CHEMBL1767136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418267 (CHEMBL1767138) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418266 (CHEMBL1767141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418265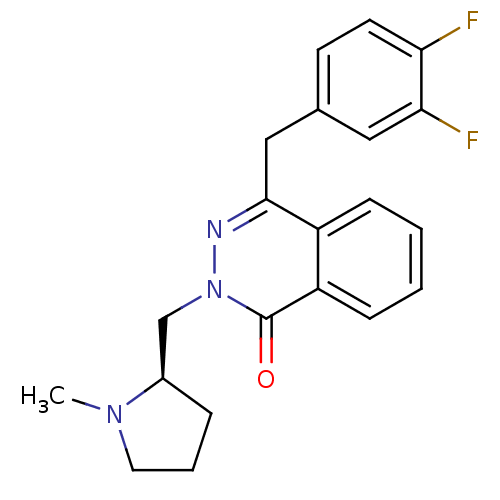 (CHEMBL1767149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418298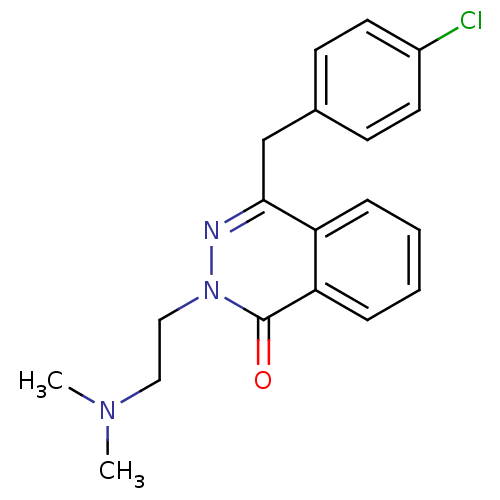 (CHEMBL1767134) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341447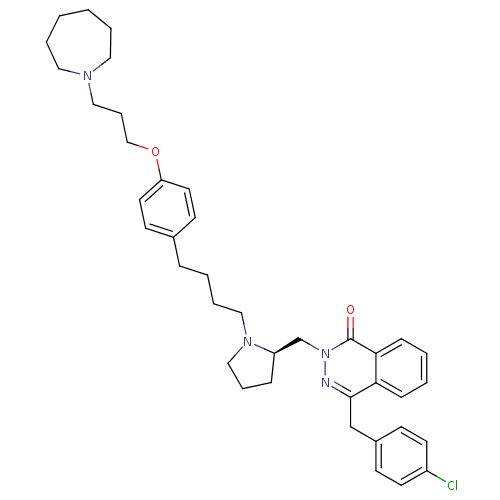 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418296 (CHEMBL1767140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418297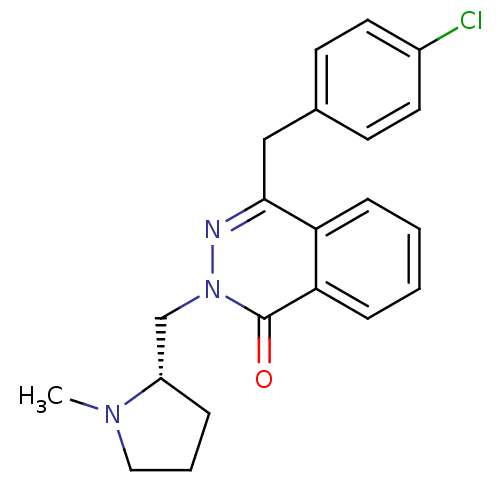 (CHEMBL1767154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205359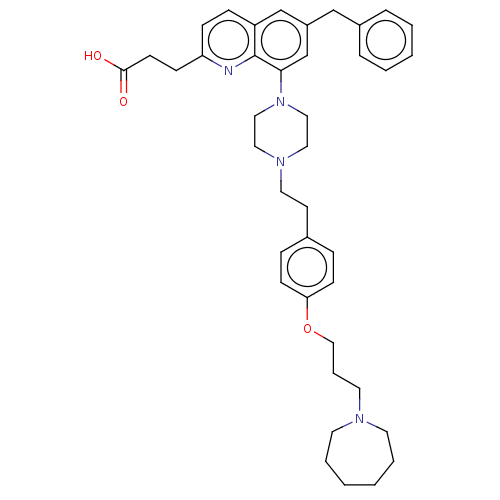 (CHEMBL3917428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205360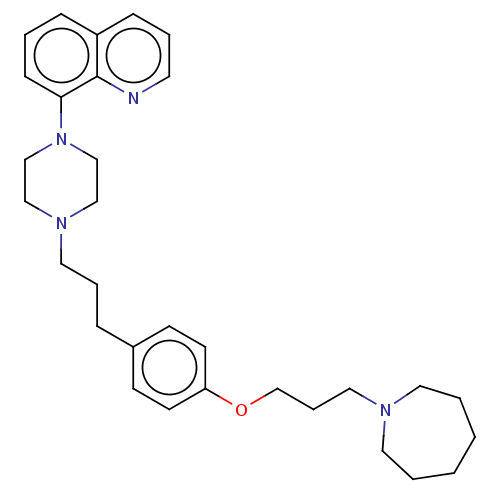 (CHEMBL3921827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418295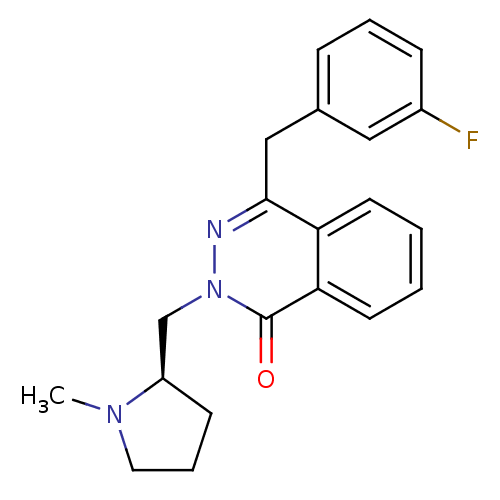 (CHEMBL1767145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205353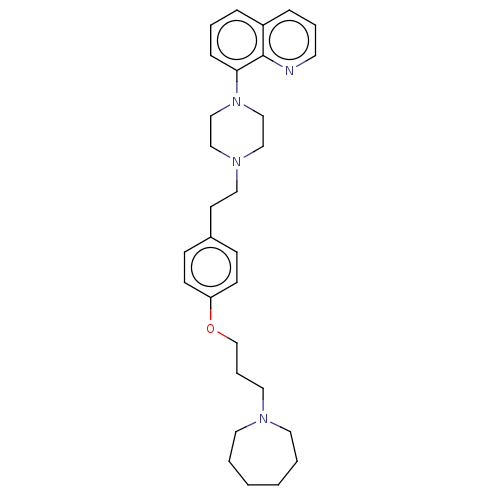 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205367 (CHEMBL3917794) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205361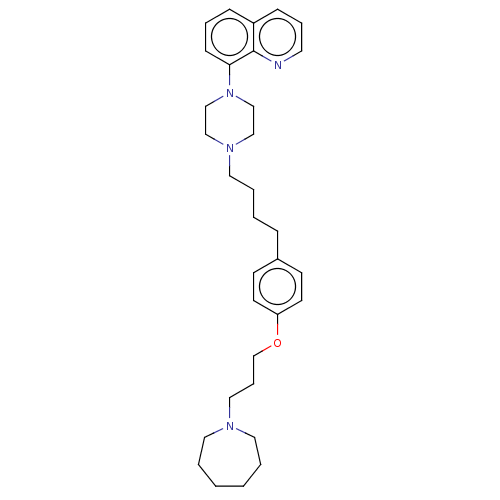 (CHEMBL3893197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418294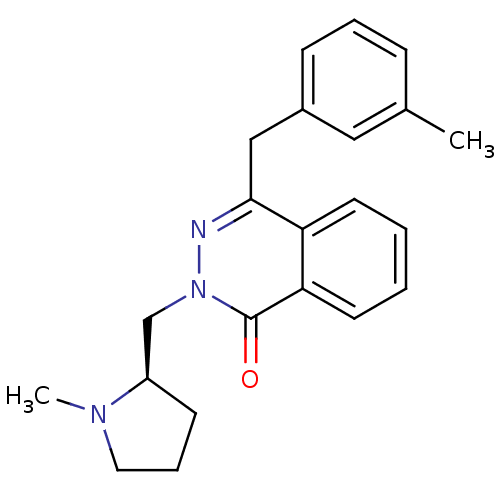 (CHEMBL1767148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205358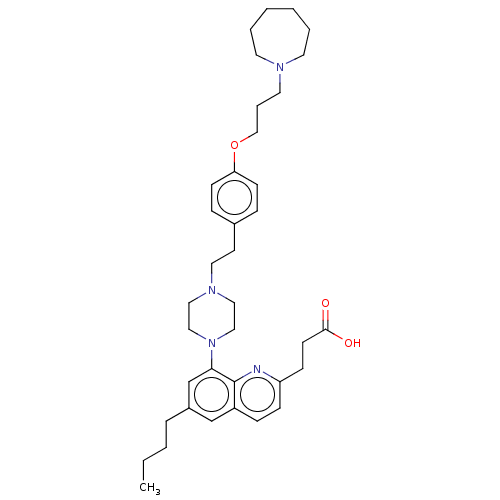 (CHEMBL3898341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50266064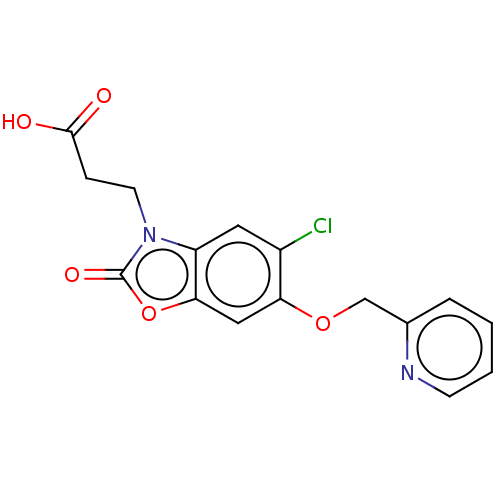 (CHEMBL4104310) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... | J Med Chem 60: 3383-3404 (2017) Article DOI: 10.1021/acs.jmedchem.7b00055 BindingDB Entry DOI: 10.7270/Q26112SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418293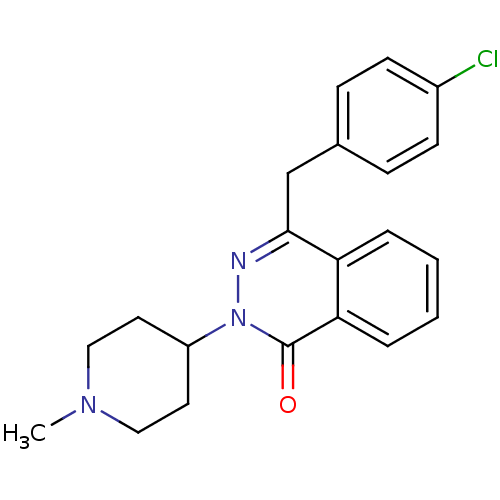 (CHEMBL1767156) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H1 receptor expressed in CHO cells assessed as inhibition of histamine-induced calcium flux preincubated for 3... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50341448 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205365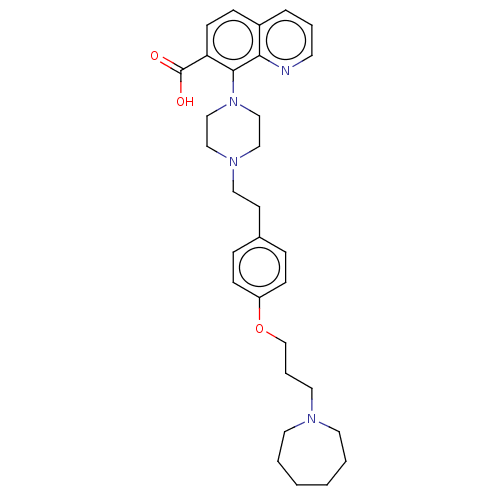 (CHEMBL3925977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205369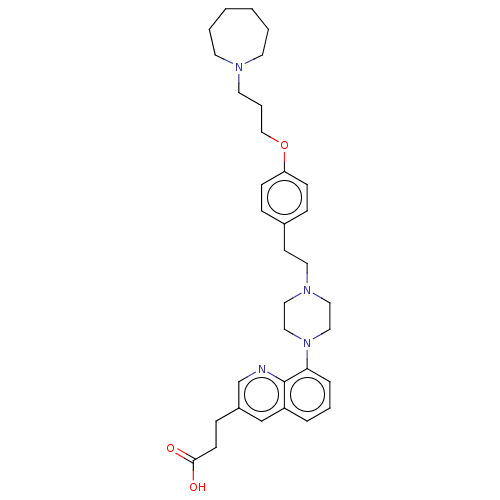 (CHEMBL3907368) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418292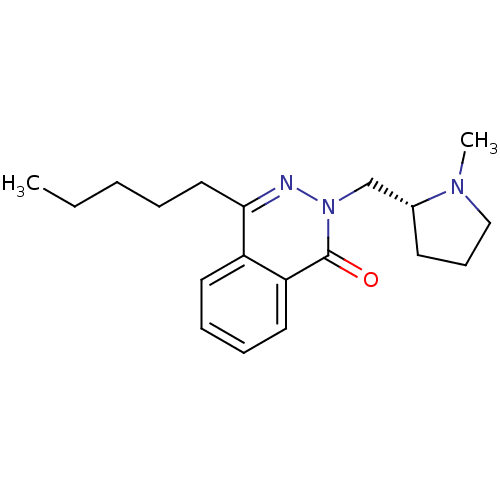 (CHEMBL1767153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418291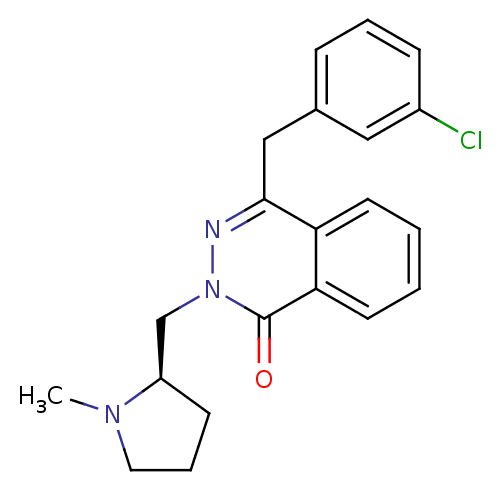 (CHEMBL1767146) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205356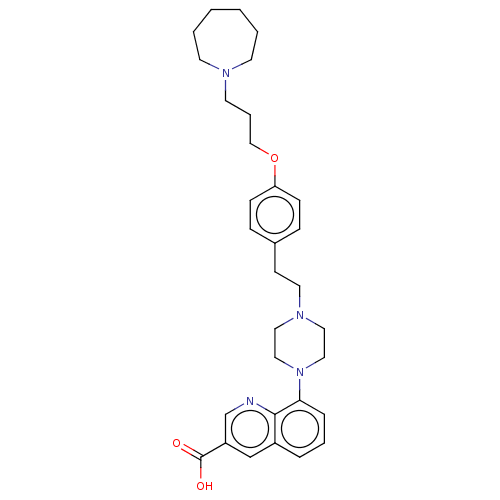 (CHEMBL3967709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205364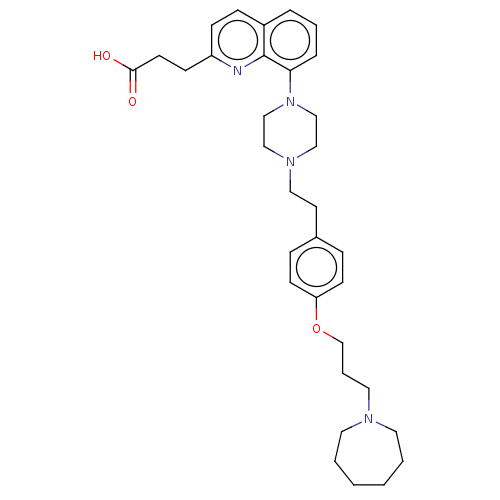 (CHEMBL3935303) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205355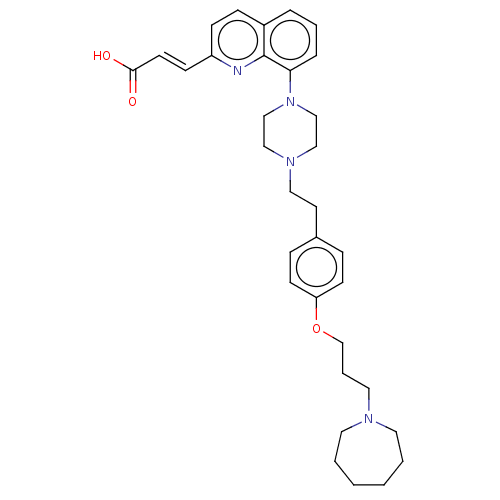 (CHEMBL3952802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205362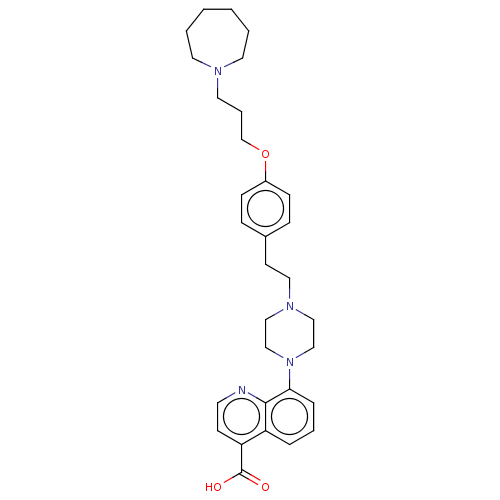 (CHEMBL3896541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205354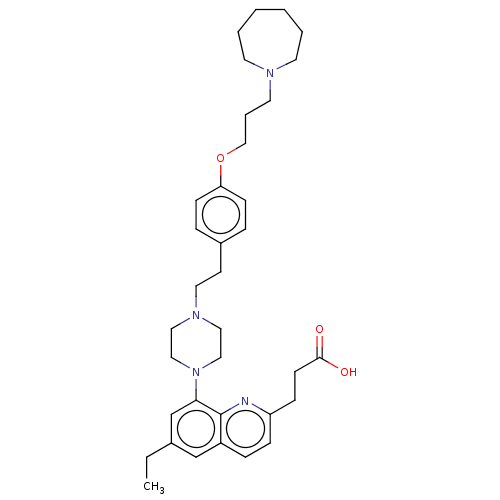 (CHEMBL3926412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418290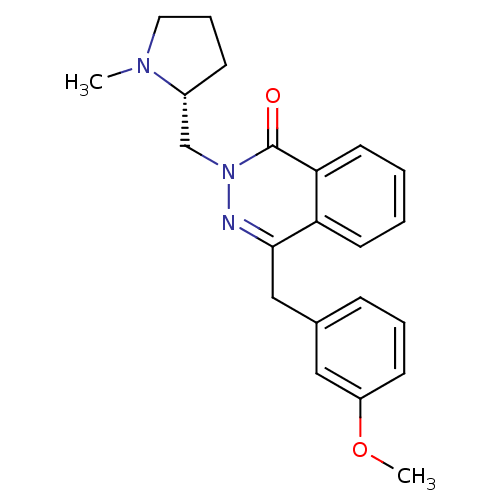 (CHEMBL1767147) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262918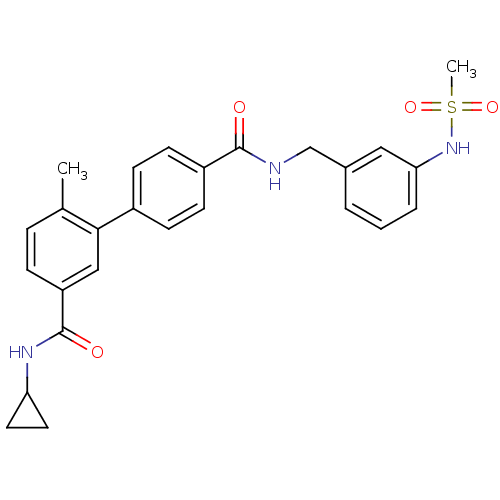 (6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205357 (CHEMBL3925332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418289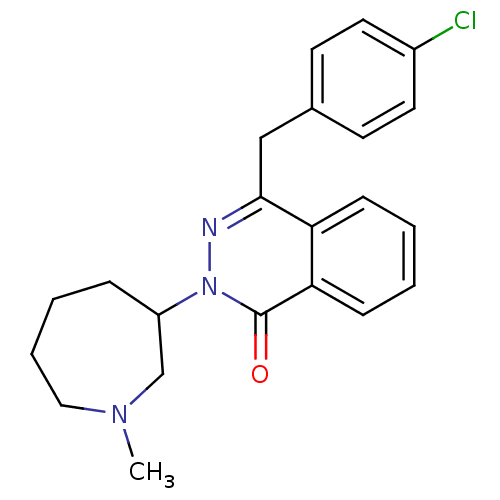 (CHEMBL1767158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262974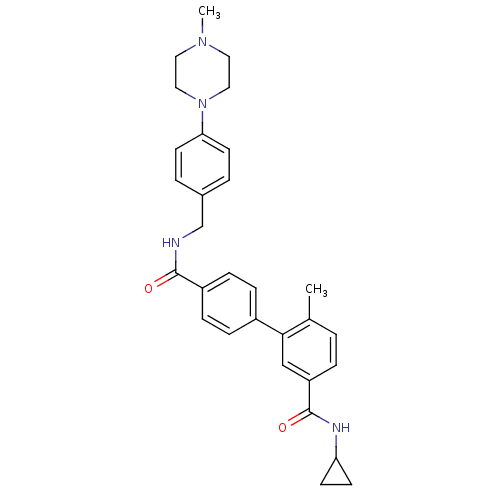 (6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50205366 (CHEMBL3953018) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human Histamine H3 receptor expressed in CHO cell membranes assessed as inhibition of histamine-induced [35S]GTPgammaS binding... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50418599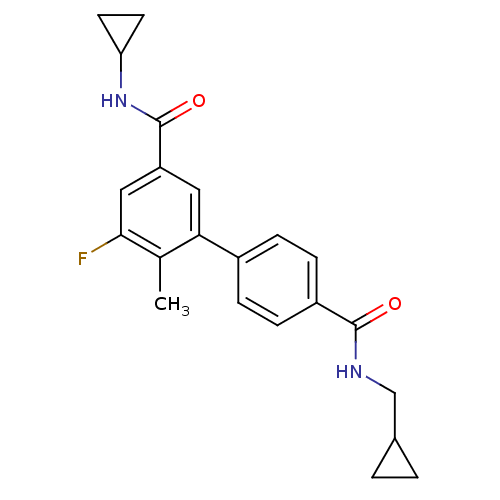 (CHEMBL1784164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged p38alpha by fluorescence polarization method | J Med Chem 52: 6257-69 (2009) Article DOI: 10.1021/jm9004779 BindingDB Entry DOI: 10.7270/Q2J967NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262917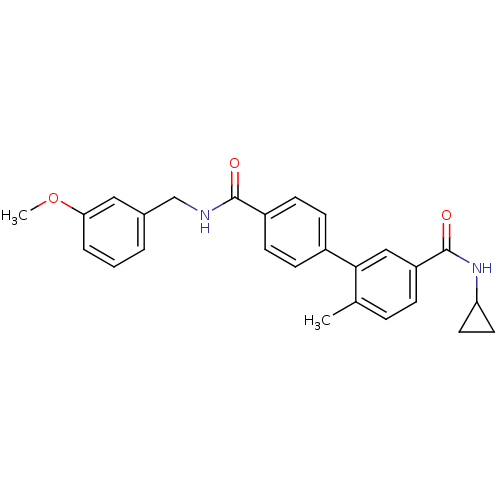 (6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262867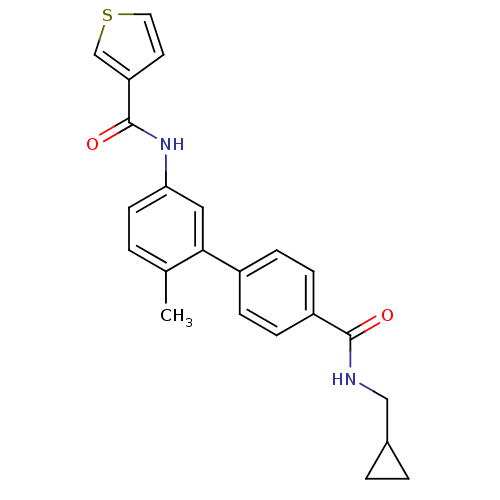 (CHEMBL476542 | Thiophene-3-carboxylic acid [4'-(cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262915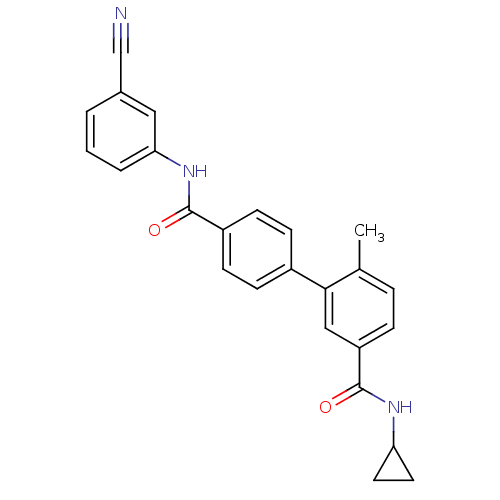 (6-Methyl-biphenyl-3,4'-dicarboxylic acid 4'-[(3-cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418288 (CHEMBL1767139) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262868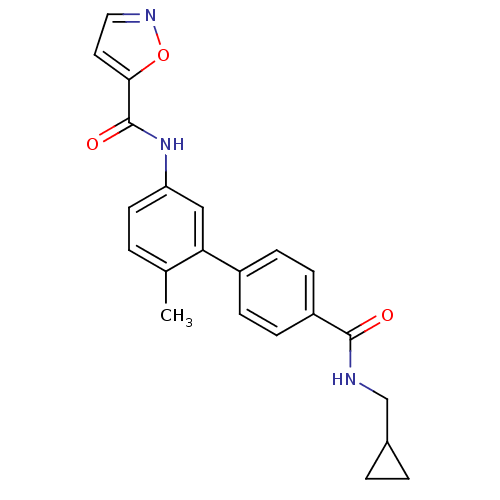 (CHEMBL476543 | Isoxazole-5-carboxylic acid [4'-(cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50418612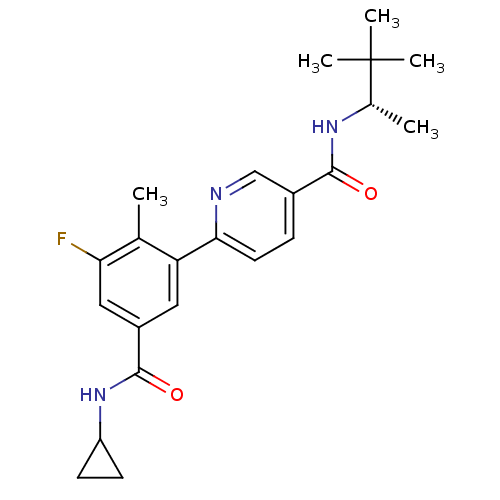 (CHEMBL1784177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged p38alpha by fluorescence polarization method | J Med Chem 52: 6257-69 (2009) Article DOI: 10.1021/jm9004779 BindingDB Entry DOI: 10.7270/Q2J967NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418287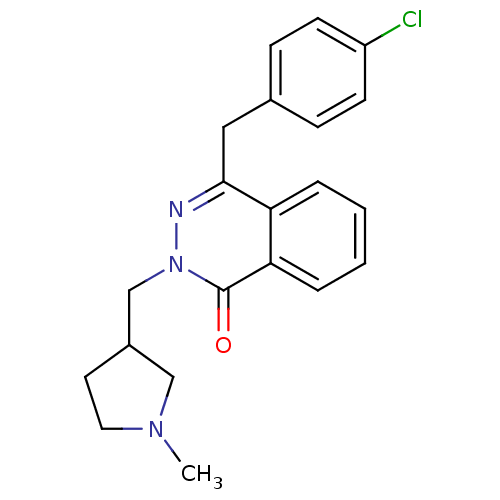 (CHEMBL1767155) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50262916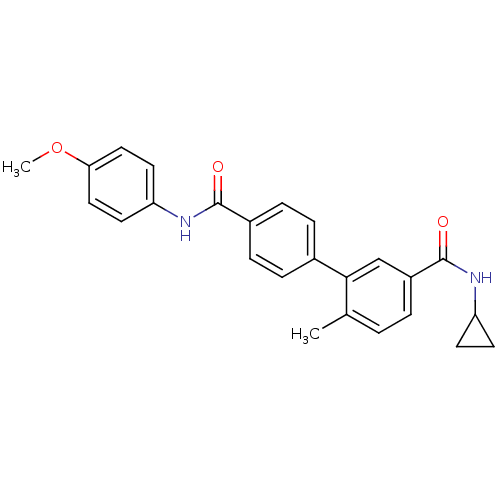 (6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... | Bioorg Med Chem Lett 18: 4428-32 (2008) Article DOI: 10.1016/j.bmcl.2008.06.048 BindingDB Entry DOI: 10.7270/Q2CR5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM35938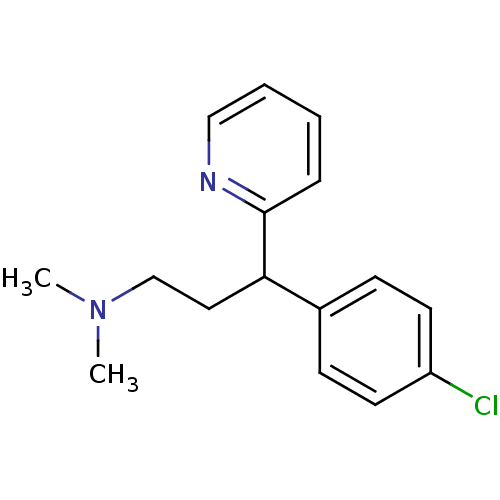 (1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50205353 (CHEMBL3947980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at adrenergic alpha1A receptor (unknown origin) expressed in Rat1 cells assessed as inhibition of phenylephrine-induced Ca2+ flux... | Bioorg Med Chem Lett 26: 5855-5859 (2016) Article DOI: 10.1016/j.bmcl.2016.11.022 BindingDB Entry DOI: 10.7270/Q2DZ0B8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50418607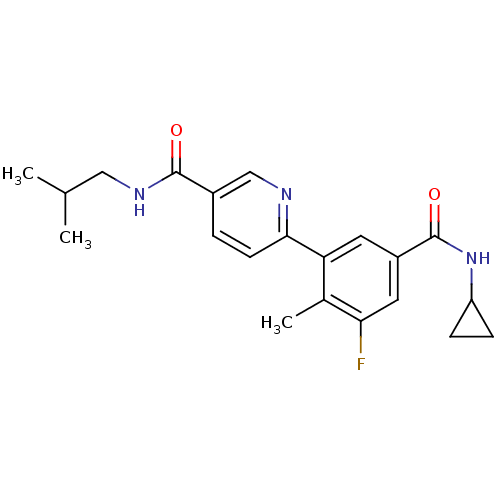 (CHEMBL1784173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged p38alpha by fluorescence polarization method | J Med Chem 52: 6257-69 (2009) Article DOI: 10.1021/jm9004779 BindingDB Entry DOI: 10.7270/Q2J967NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1228 total ) | Next | Last >> |