 Found 310 hits with Last Name = 'hostetler' and Initial = 'e'
Found 310 hits with Last Name = 'hostetler' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
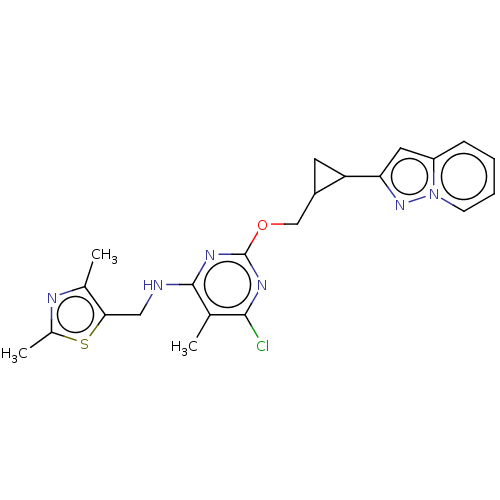
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
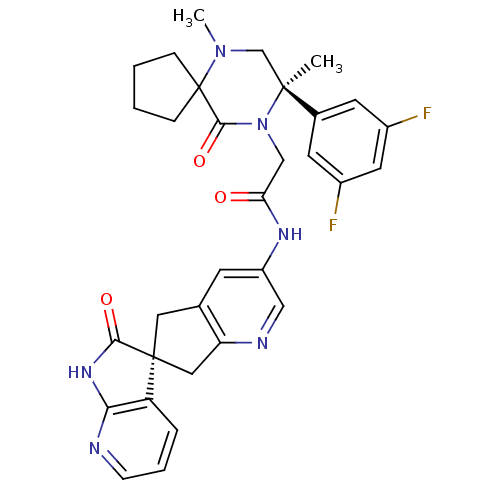
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
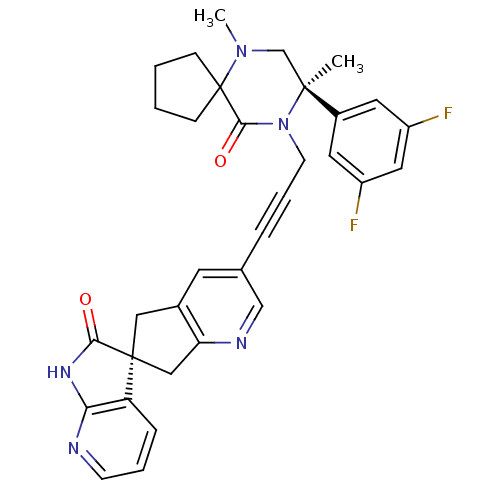
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
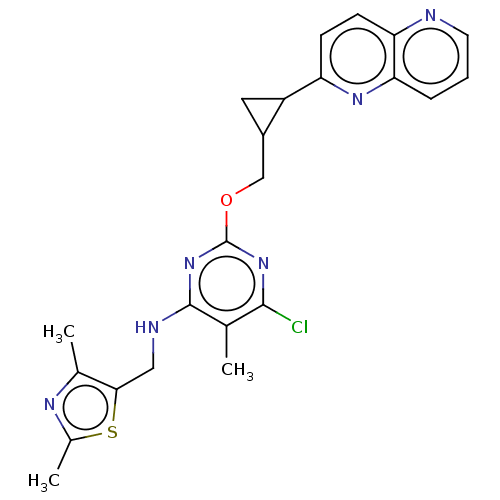
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
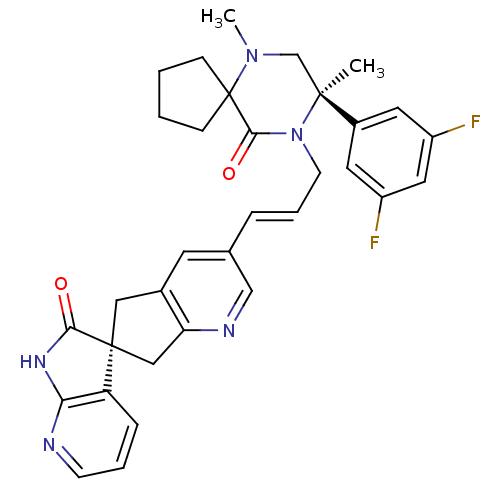
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
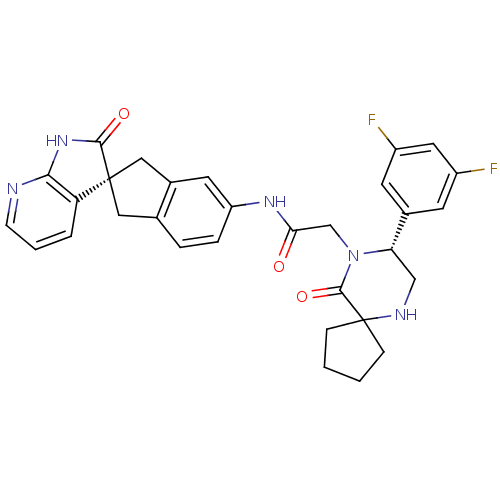
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
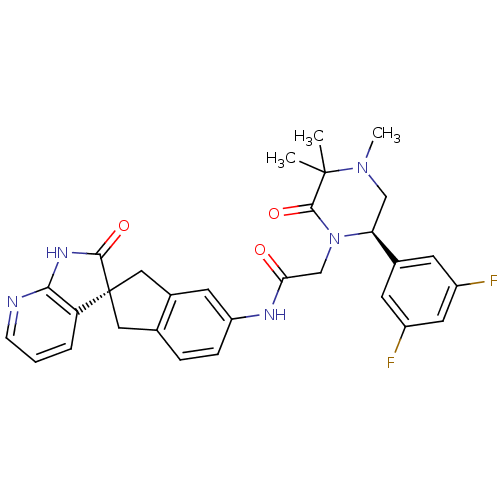
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135609

(US8846000, G-2)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C30H27N5O4/c1-17(2)39-25-7-5-6-22-27(25)30(38)34(28(22)36)13-12-26-32-23-14-18(3)8-11-21(23)29(37)35(26)20-10-9-19-16-31-33(4)24(19)15-20/h5-11,14-17H,12-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823
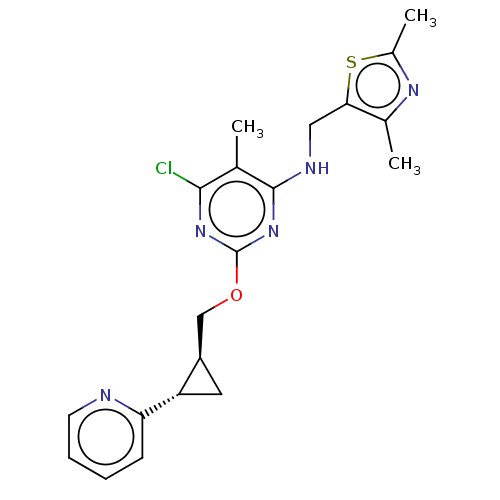
(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440793
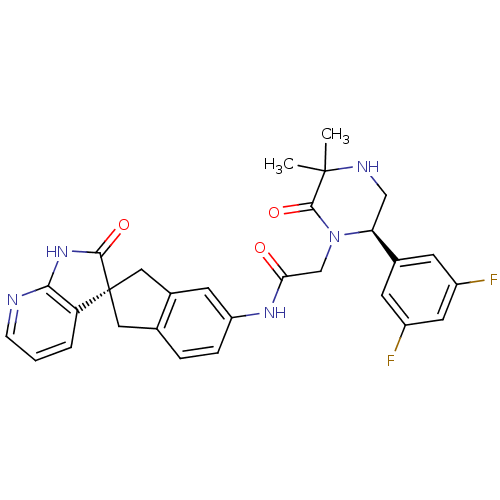
(CHEMBL2431254)Show SMILES CC1(C)NC[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H27F2N5O3/c1-28(2)27(39)36(23(14-33-28)17-8-19(30)11-20(31)9-17)15-24(37)34-21-6-5-16-12-29(13-18(16)10-21)22-4-3-7-32-25(22)35-26(29)38/h3-11,23,33H,12-15H2,1-2H3,(H,34,37)(H,32,35,38)/t23-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385314
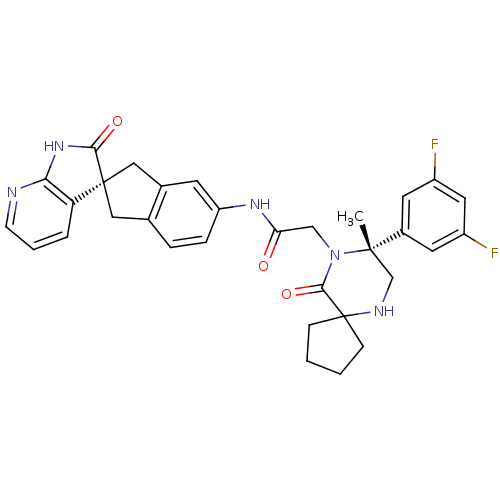
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440786
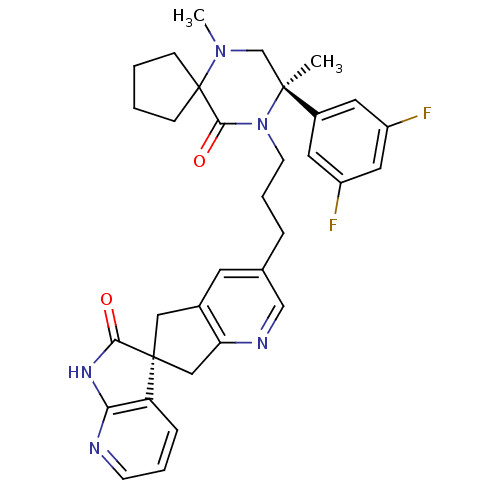
(CHEMBL2431251)Show SMILES CN1C[C@](C)(N(CCCc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H35F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,6-7,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135597

(US8846000, 1-13)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1ccc3cc[nH]c3c1)c2=O Show InChI InChI=1S/C30H26N4O5/c1-17(2)39-25-6-4-5-22-27(25)30(37)33(28(22)35)14-12-26-32-24-16-20(38-3)9-10-21(24)29(36)34(26)19-8-7-18-11-13-31-23(18)15-19/h4-11,13,15-17,31H,12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440790
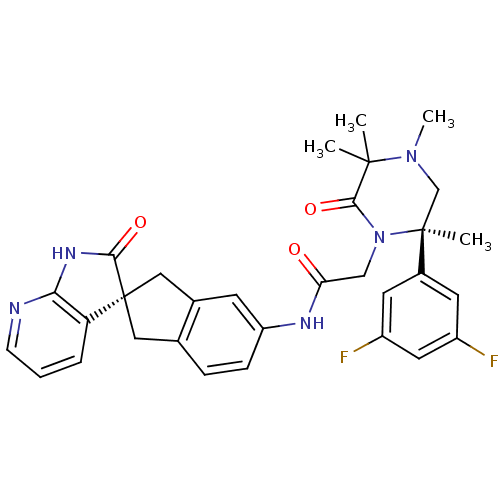
(CHEMBL2431247)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N5O3/c1-29(2)28(41)38(30(3,17-37(29)4)20-11-21(32)13-22(33)12-20)16-25(39)35-23-8-7-18-14-31(15-19(18)10-23)24-6-5-9-34-26(24)36-27(31)40/h5-13H,14-17H2,1-4H3,(H,35,39)(H,34,36,40)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
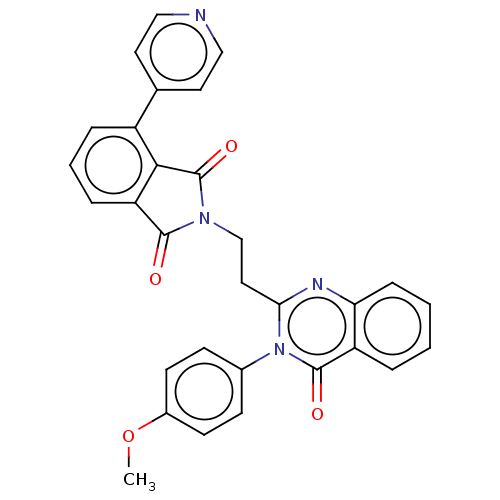
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600

(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135598

(US8846000, 1-14)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1cccc(C)c1)c2=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-10-6-9-22-26(24)29(35)31(27(22)33)14-13-25-30-23-16-20(36-4)11-12-21(23)28(34)32(25)19-8-5-7-18(3)15-19/h5-12,15-17H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440785
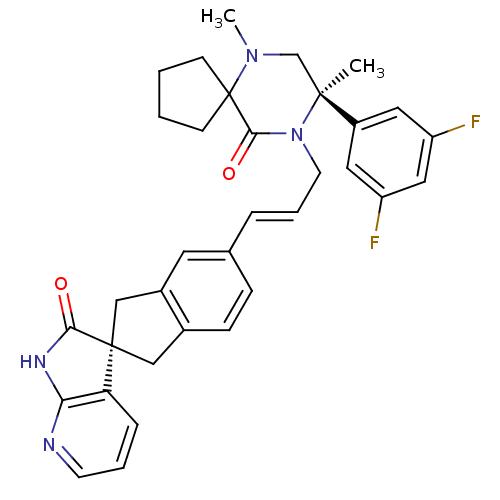
(CHEMBL2431252)Show SMILES CN1C[C@](C)(N(C\C=C\c2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H34F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/b7-6+/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135606

(US8846000, 1-24)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-7-5-6-22-26(24)29(35)31(27(22)33)15-14-25-30-23-16-18(3)8-13-21(23)28(34)32(25)19-9-11-20(36-4)12-10-19/h5-13,16-17H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135608

(US8846000, G-1)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cn[nH]c4c3)C(=O)c12 Show InChI InChI=1S/C29H25N5O4/c1-16(2)38-24-6-4-5-21-26(24)29(37)33(27(21)35)12-11-25-31-23-13-17(3)7-10-20(23)28(36)34(25)19-9-8-18-15-30-32-22(18)14-19/h4-10,13-16H,11-12H2,1-3H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440792

(CHEMBL2431255)Show SMILES CC1(C)NC[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-28(2)27(40)37(29(3,16-34-28)19-10-20(31)12-21(32)11-19)15-24(38)35-22-7-6-17-13-30(14-18(17)9-22)23-5-4-8-33-25(23)36-26(30)39/h4-12,34H,13-16H2,1-3H3,(H,35,38)(H,33,36,39)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440783
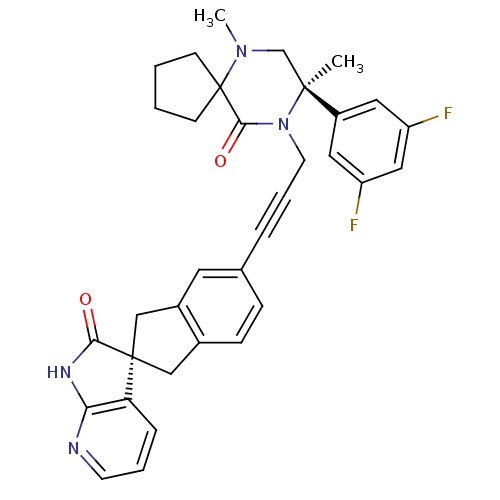
(CHEMBL2429882)Show SMILES CN1C[C@](C)(N(CC#Cc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H32F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM314813

((3aR,5S,6S,7R,7aR)-5-((R)-1-(4- benzylbenzyloxy)-2...)Show SMILES CNC1=N[C@H]2[C@H](O[C@H]([C@@H](OCc3ccc(Cc4ccccc4)cc3)C(F)(F)F)[C@@H](O)[C@@H]2O)S1 |t:2| Show InChI InChI=1S/C23H25F3N2O4S/c1-27-22-28-16-17(29)18(30)19(32-21(16)33-22)20(23(24,25)26)31-12-15-9-7-14(8-10-15)11-13-5-3-2-4-6-13/h2-10,16-21,29-30H,11-12H2,1H3,(H,27,28)/t16-,17-,18+,19+,20-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALECTOS THERAPEUTICS, INC.; MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... |
US Patent US9611275 (2017)
BindingDB Entry DOI: 10.7270/Q2833V4D |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281656
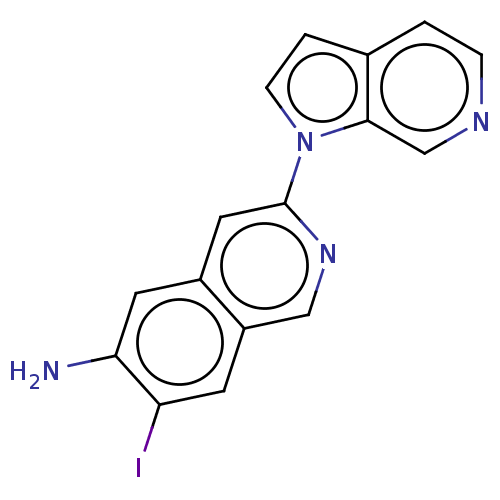
(US10022461, Compound 126)Show InChI InChI=1S/C16H11IN4/c17-13-5-12-8-20-16(7-11(12)6-14(13)18)21-4-2-10-1-3-19-9-15(10)21/h1-9H,18H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.180 | -55.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135596

(US8846000, 1-12)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OCCF)cc3)C(=O)c12 Show InChI InChI=1S/C29H26FN3O5/c1-18(2)38-24-9-5-7-22-26(24)29(36)32(27(22)34)16-14-25-31-23-8-4-3-6-21(23)28(35)33(25)19-10-12-20(13-11-19)37-17-15-30/h3-13,18H,14-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM314809
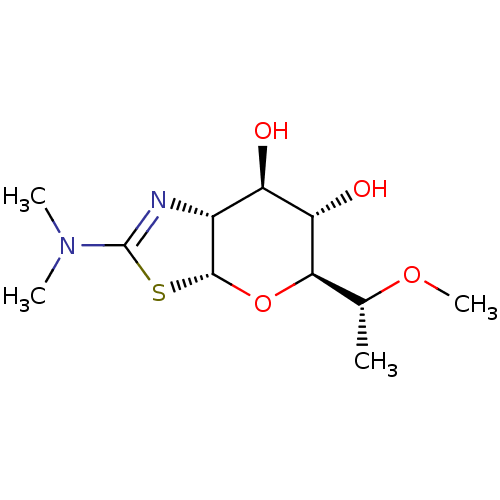
((3aR,5S,6S,7R,7aR)-2-(dimethylamino)-5-((R)-1-meth...)Show SMILES CO[C@H](C)[C@H]1O[C@@H]2SC(=N[C@@H]2[C@@H](O)[C@@H]1O)N(C)C |c:8| Show InChI InChI=1S/C11H20N2O4S/c1-5(16-4)9-8(15)7(14)6-10(17-9)18-11(12-6)13(2)3/h5-10,14-15H,1-4H3/t5-,6-,7-,8+,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALECTOS THERAPEUTICS, INC.; MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... |
US Patent US9611275 (2017)
BindingDB Entry DOI: 10.7270/Q2833V4D |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281558
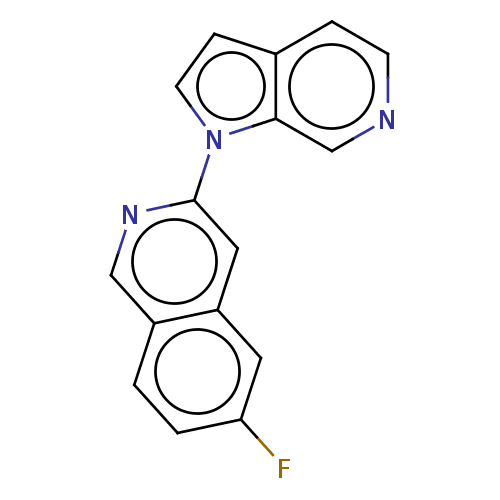
(US10022461, Compound 9)Show InChI InChI=1S/C16H10FN3/c17-14-2-1-12-9-19-16(8-13(12)7-14)20-6-4-11-3-5-18-10-15(11)20/h1-10H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281645
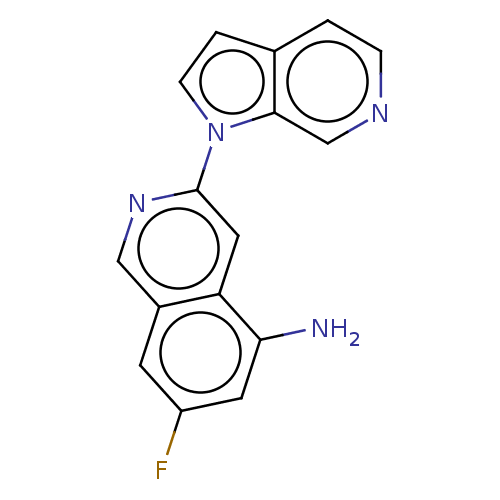
(US10022461, Compound 115)Show InChI InChI=1S/C16H11FN4/c17-12-5-11-8-20-16(7-13(11)14(18)6-12)21-4-2-10-1-3-19-9-15(10)21/h1-9H,18H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.224 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147134
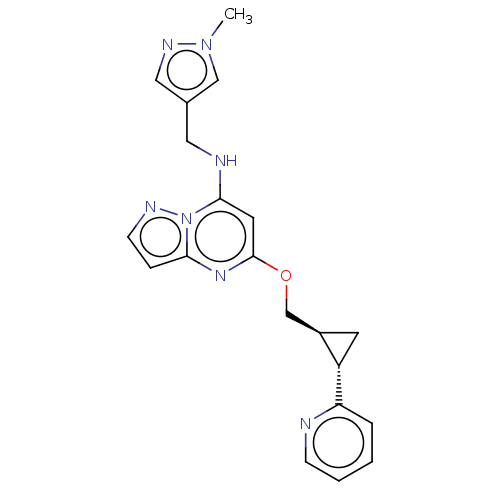
(US8957077, J-5)Show SMILES Cn1cc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)cn1 |r| Show InChI InChI=1S/C20H21N7O/c1-26-12-14(11-24-26)10-22-19-9-20(25-18-5-7-23-27(18)19)28-13-15-8-16(15)17-4-2-3-6-21-17/h2-7,9,11-12,15-16,22H,8,10,13H2,1H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135613
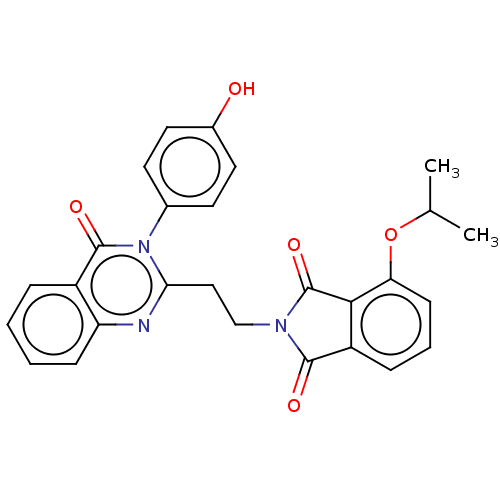
(US8846000, D-6)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(O)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-16(2)35-22-9-5-7-20-24(22)27(34)29(25(20)32)15-14-23-28-21-8-4-3-6-19(21)26(33)30(23)17-10-12-18(31)13-11-17/h3-13,16,31H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281561
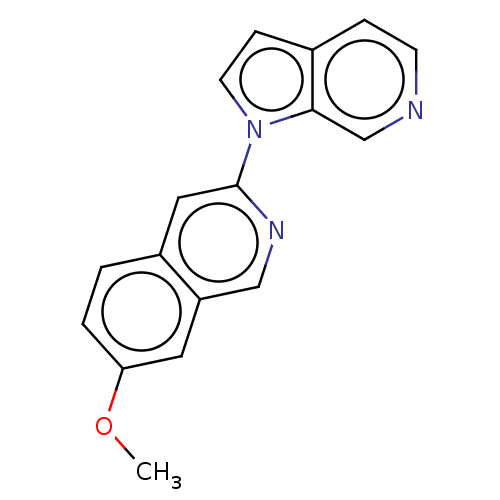
(US10022461, Compound 13)Show InChI InChI=1S/C17H13N3O/c1-21-15-3-2-13-9-17(19-10-14(13)8-15)20-7-5-12-4-6-18-11-16(12)20/h2-11H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 0.290 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281560
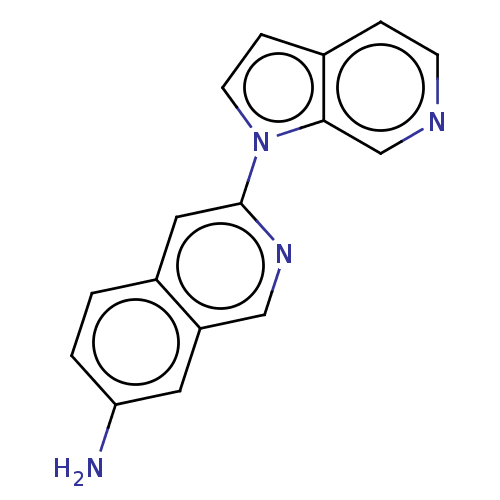
(US10022461, Compound 12)Show InChI InChI=1S/C16H12N4/c17-14-2-1-12-8-16(19-9-13(12)7-14)20-6-4-11-3-5-18-10-15(11)20/h1-10H,17H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 0.290 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281633

(US10022461, Compound 103)Show InChI InChI=1S/C17H13N3O/c1-21-16-4-2-3-13-9-17(19-10-14(13)16)20-8-6-12-5-7-18-11-15(12)20/h2-11H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281563
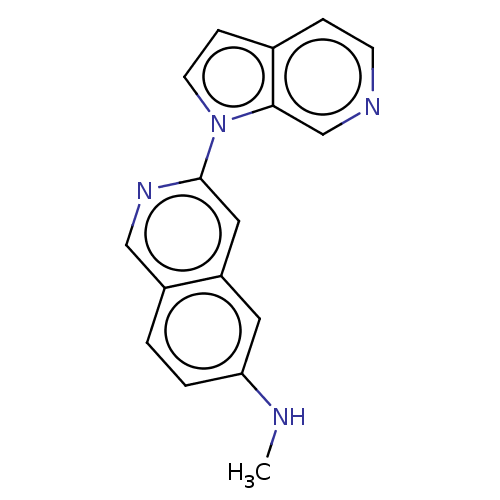
(US10022461, Compound 14-1)Show InChI InChI=1S/C17H14N4/c1-18-15-3-2-13-10-20-17(9-14(13)8-15)21-7-5-12-4-6-19-11-16(12)21/h2-11,18H,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281609
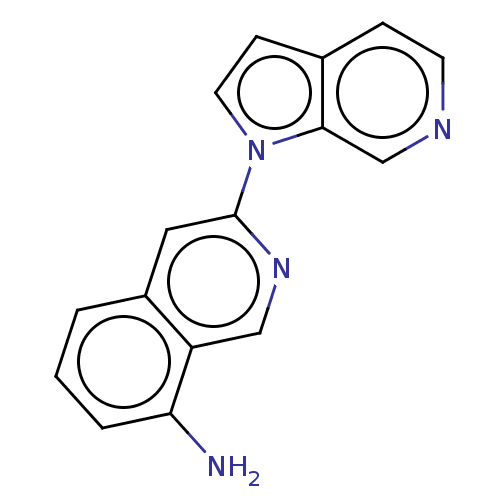
(US10022461, Compound 59)Show InChI InChI=1S/C16H12N4/c17-14-3-1-2-12-8-16(19-9-13(12)14)20-7-5-11-4-6-18-10-15(11)20/h1-10H,17H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595
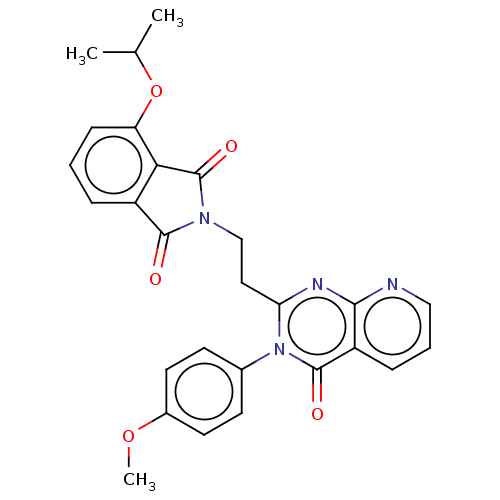
(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281635
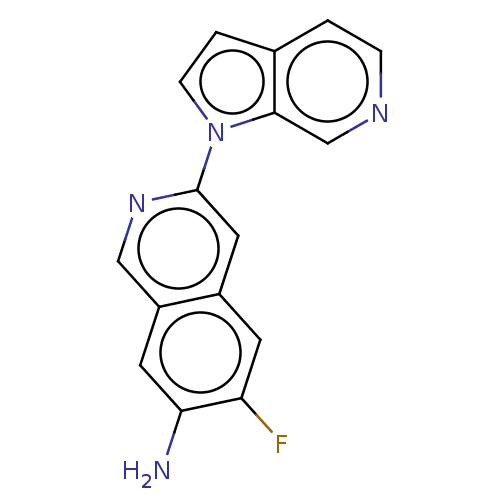
(US10022461, Compound 105)Show InChI InChI=1S/C16H11FN4/c17-13-5-11-7-16(20-8-12(11)6-14(13)18)21-4-2-10-1-3-19-9-15(10)21/h1-9H,18H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.383 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281559
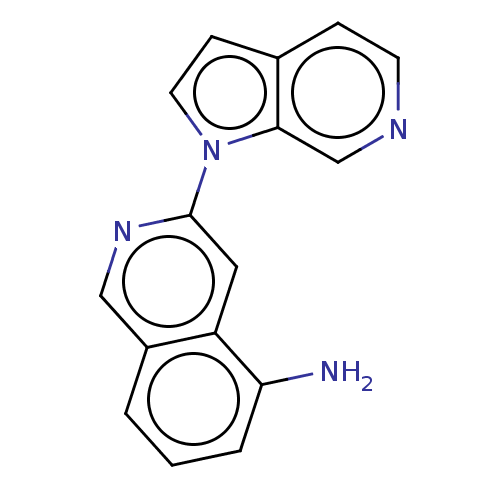
(US10022461, Compound 11-5)Show InChI InChI=1S/C16H12N4/c17-14-3-1-2-12-9-19-16(8-13(12)14)20-7-5-11-4-6-18-10-15(11)20/h1-10H,17H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500537
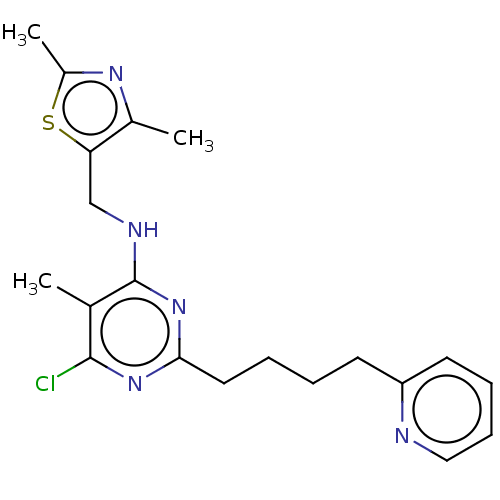
(CHEMBL3746162)Show InChI InChI=1S/C20H24ClN5S/c1-13-19(21)25-18(10-5-4-8-16-9-6-7-11-22-16)26-20(13)23-12-17-14(2)24-15(3)27-17/h6-7,9,11H,4-5,8,10,12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281565
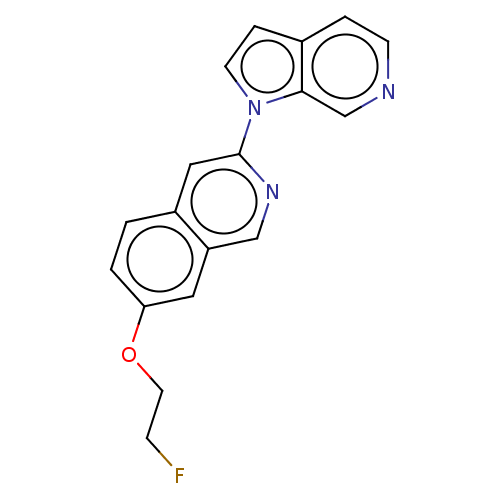
(US10022461, Compound 16)Show InChI InChI=1S/C18H14FN3O/c19-5-8-23-16-2-1-14-10-18(21-11-15(14)9-16)22-7-4-13-3-6-20-12-17(13)22/h1-4,6-7,9-12H,5,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.410 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM281639
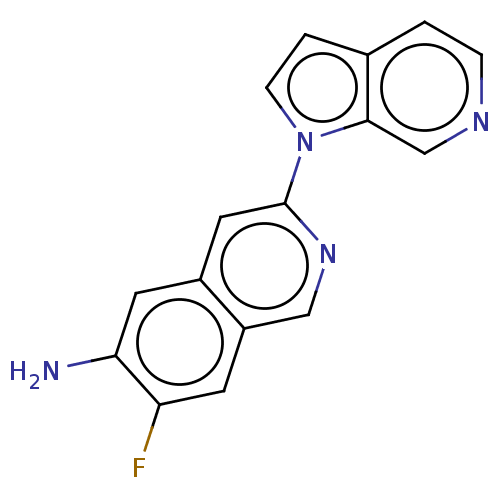
(US10022461, Compound 109)Show InChI InChI=1S/C16H11FN4/c17-13-5-12-8-20-16(7-11(12)6-14(13)18)21-4-2-10-1-3-19-9-15(10)21/h1-9H,18H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.423 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
For displacement tau binding assay, unlabeled test compounds were dissolved in DMSO at 1 mM. Dilutions of tests compounds to various concentrations w... |
US Patent US10022461 (2018)
BindingDB Entry DOI: 10.7270/Q2959KK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data