

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024714 (2-(4-Carboxymethyl-5-oxo-3-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023299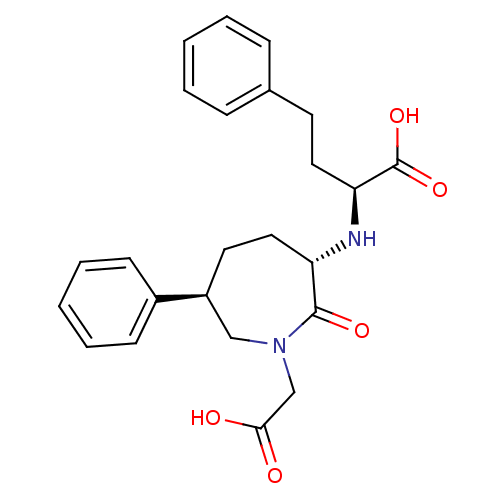 (2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024710 (2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023296 (2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295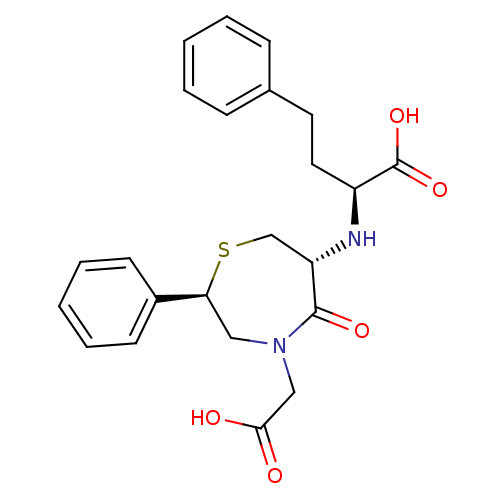 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024713 (2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023300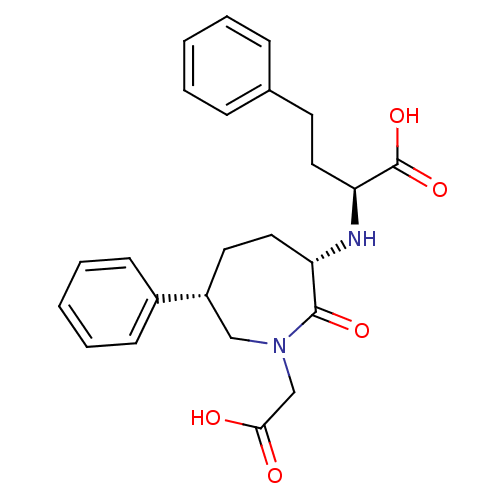 (2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011192 ((R)-1-[(S)-2-((S)-1-Carboxy-3-phenyl-propylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023302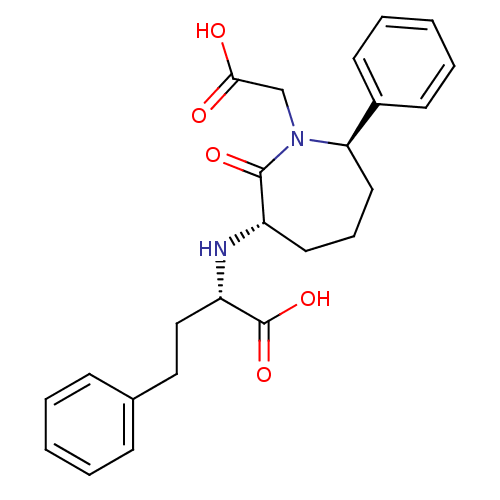 (2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50366241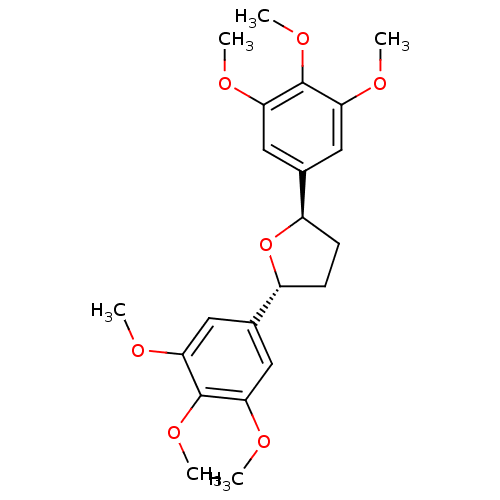 (CHEMBL297624 | L-652731) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055488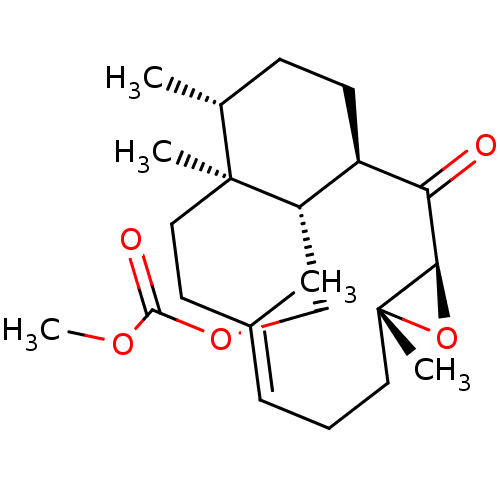 (CHEMBL148491 | Carbonic acid methyl ester (E)-(1R,...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055491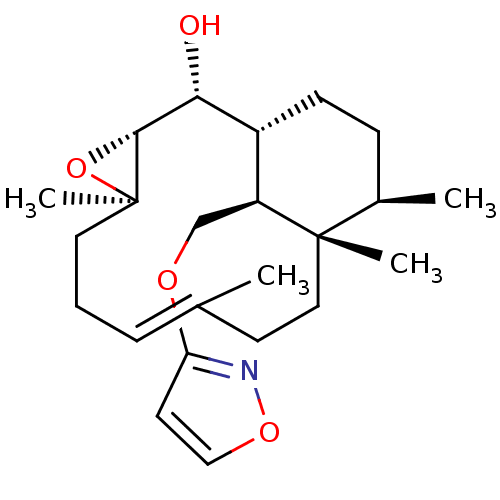 ((E)-(1R,2R,3R,5R,12S,13R,16S)-16-(Isoxazol-3-yloxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023303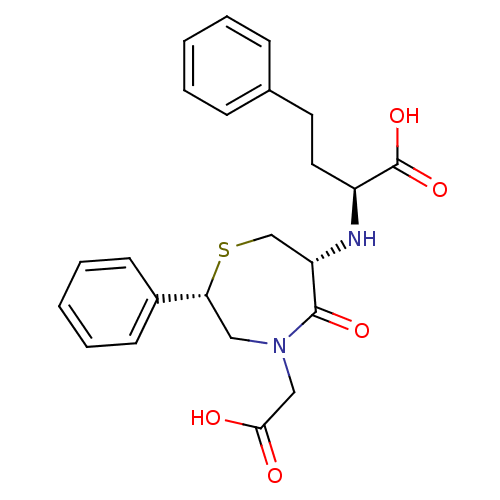 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023303 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055482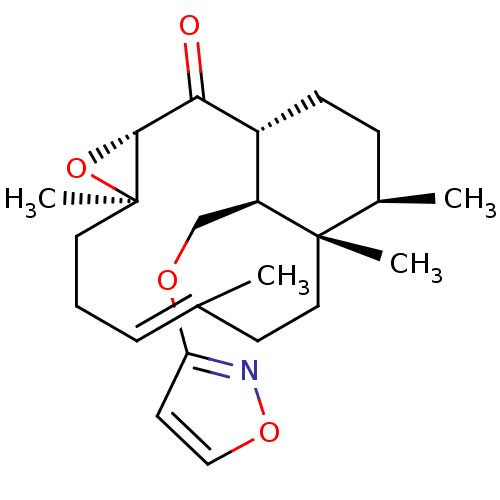 ((E)-(1R,3S,5R,12S,13R,16S)-16-(Isoxazol-3-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024712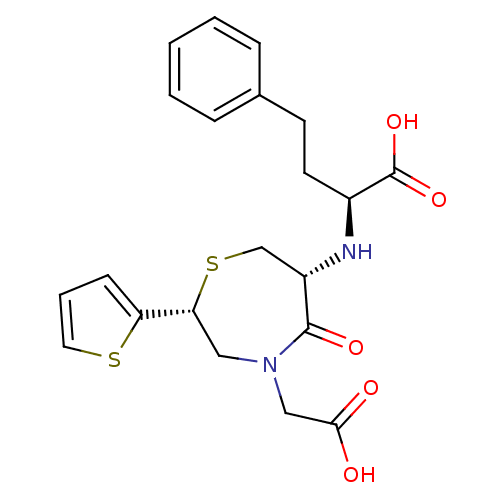 (2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023301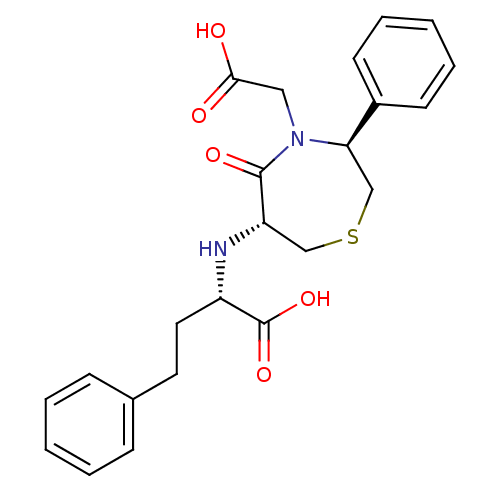 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023301 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055479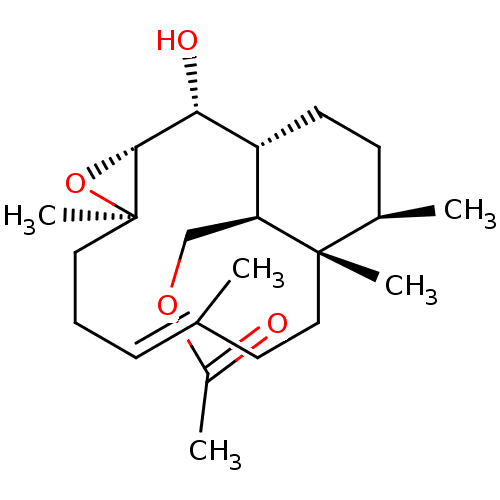 (Acetic acid (E)-(1R,2R,3R,5R,12S,13R,16S)-2-hydrox...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024711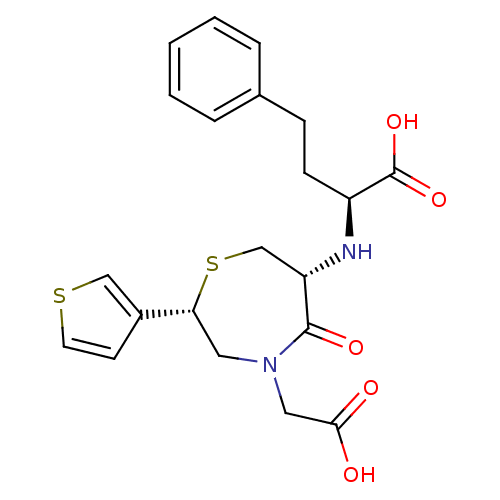 (2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055489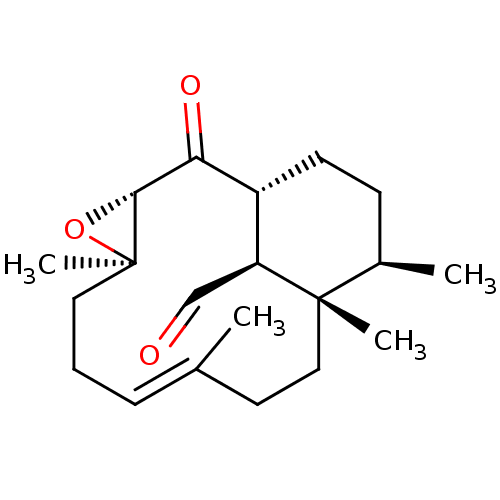 ((E)-(1R,3S,5R,12S,13R,16S)-5,9,12,13-Tetramethyl-2...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055493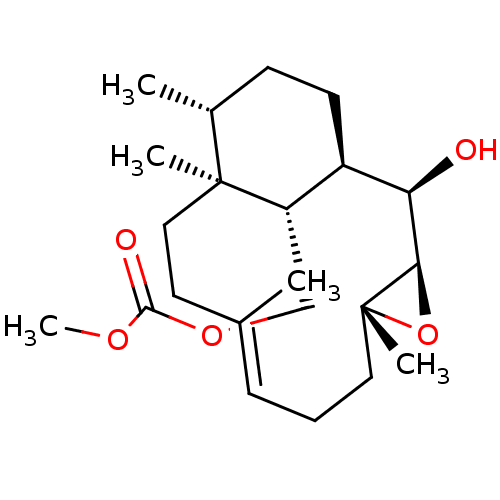 (CHEMBL148549 | Carbonic acid (E)-(1R,2R,3R,5R,12S,...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055495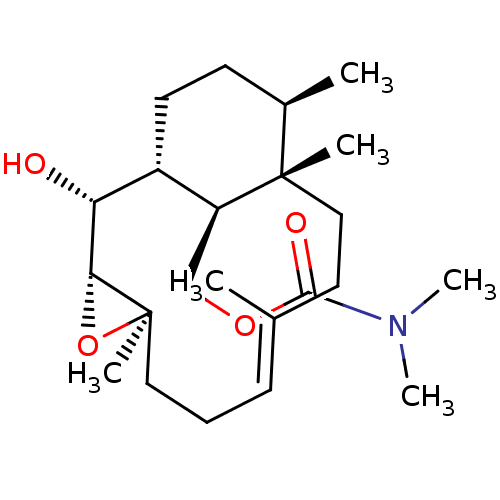 (CHEMBL151075 | Dimethyl-carbamic acid (E)-(1R,2R,3...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055483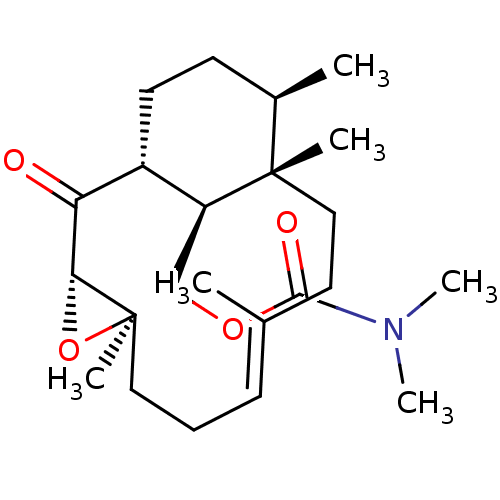 (CHEMBL151310 | Dimethyl-carbamic acid (E)-(1R,3S,5...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055492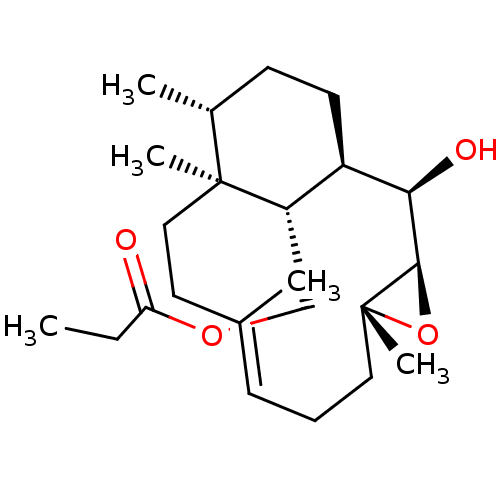 (CHEMBL147337 | Propionic acid (E)-(1R,2R,3R,5R,12S...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055487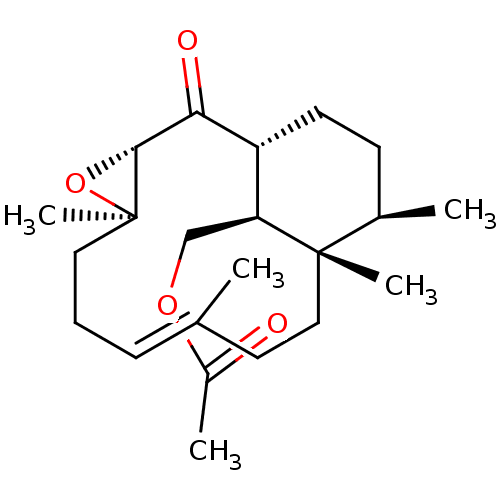 (Acetic acid (E)-(1R,3S,5R,12S,13R,16S)-5,9,12,13-t...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055484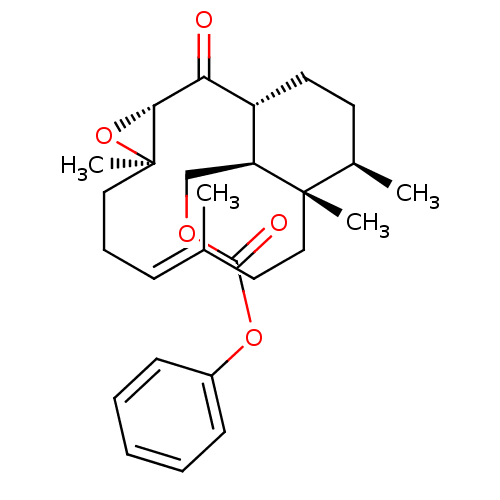 (CHEMBL421954 | Carbonic acid phenyl ester (E)-(1R,...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055494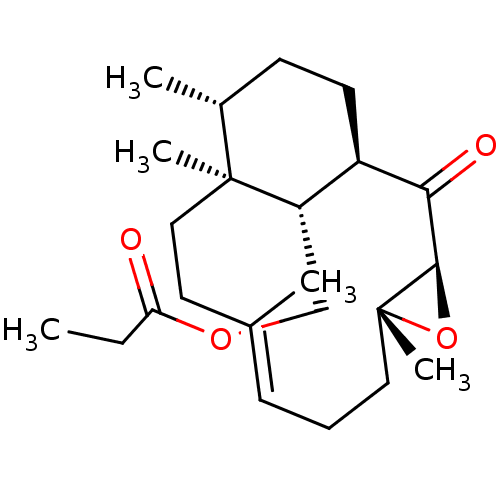 (CHEMBL147079 | Propionic acid (E)-(1R,3S,5R,12S,13...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055480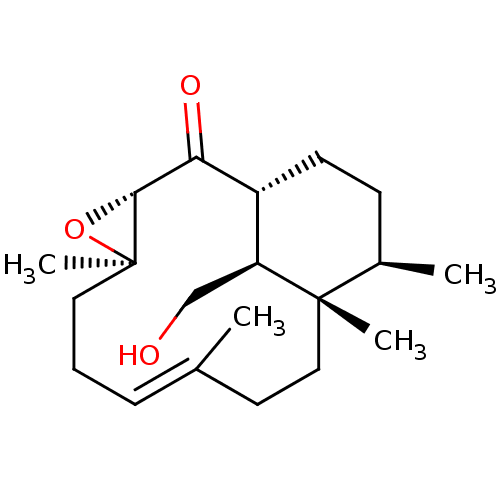 ((E)-(1R,3S,5R,12S,13R,16S)-16-Hydroxymethyl-5,9,12...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055485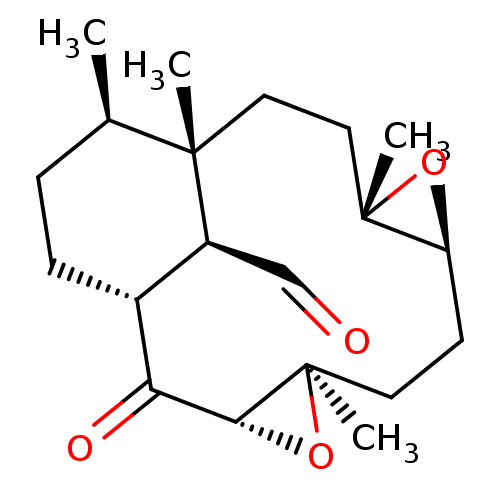 (16-(Isoxazol-3-yloxymethyl)-5,9,12,13-tetramethyl-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055481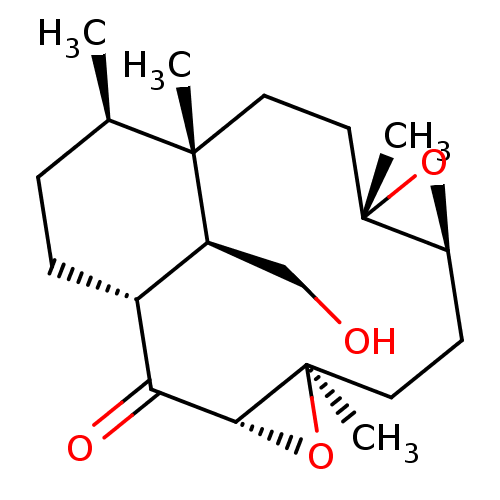 (16-(Isoxazol-3-yloxymethyl)-5,9,12,13-tetramethyl-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50055486 ((1R,3S,5R,8S,9S,12S,13R,16S)-8,9-Dihydroxy-16-hydr...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibiting Platelet activating factor receptor by radioreceptor binding assay using rabbit platelets and [3H]-PAF | J Med Chem 39: 5281-4 (1997) Article DOI: 10.1021/jm950640q BindingDB Entry DOI: 10.7270/Q2FF3T06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||