

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287890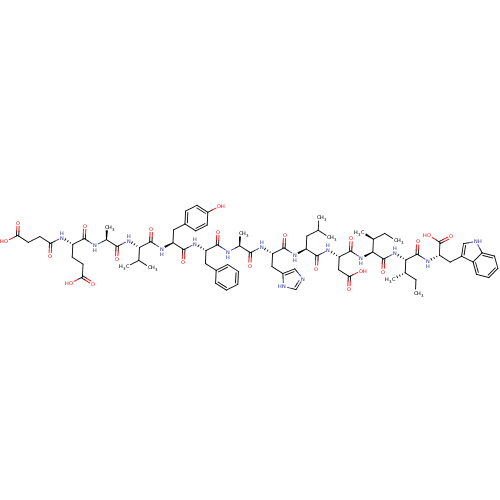 (CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287885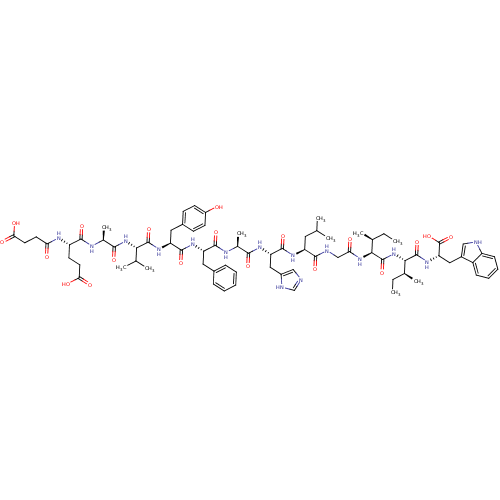 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031962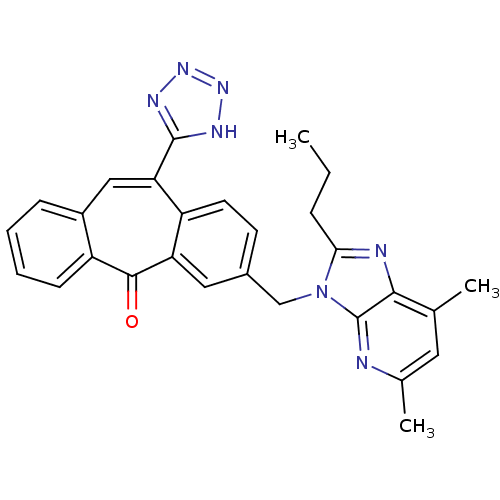 (3-(5,7-Dimethyl-2-propyl-imidazo[4,5-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031954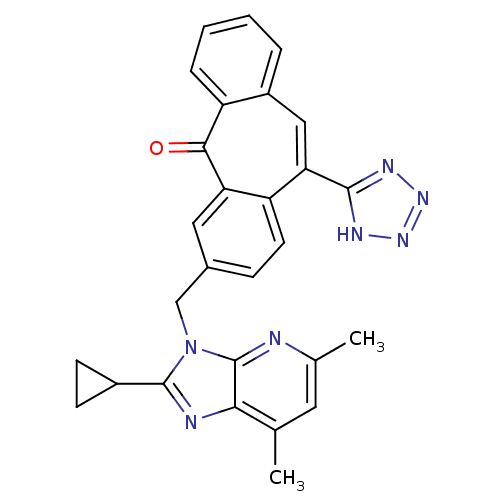 (3-(2-Cyclopropyl-5,7-dimethyl-imidazo[4,5-b]pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031953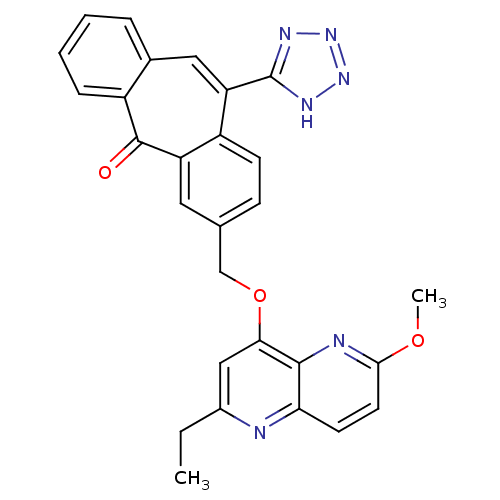 (3-(2-Ethyl-6-methoxy-[1,5]naphthyridin-4-yloxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031957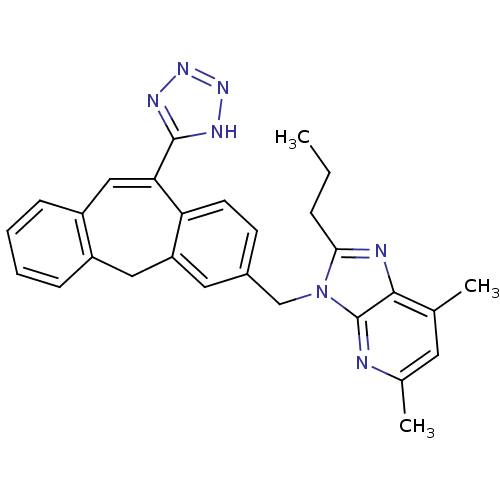 (5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-5H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031960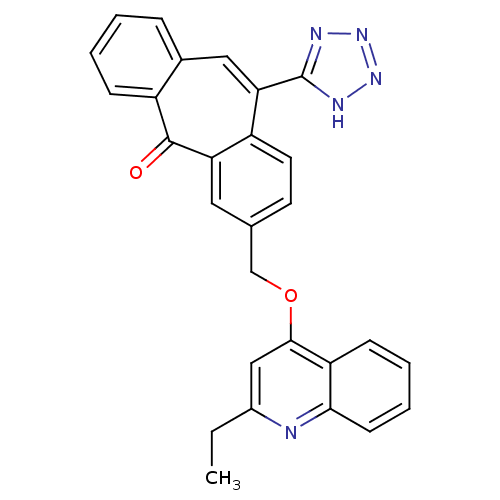 (3-(2-Ethyl-quinolin-4-yloxymethyl)-11-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031961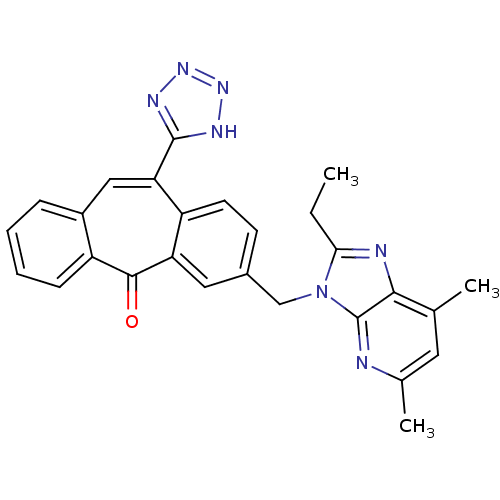 (3-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031955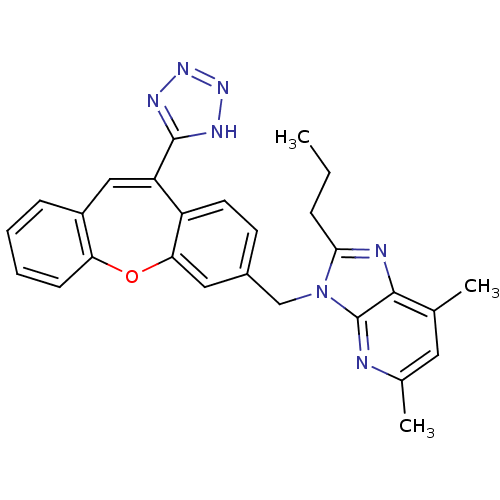 (5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287883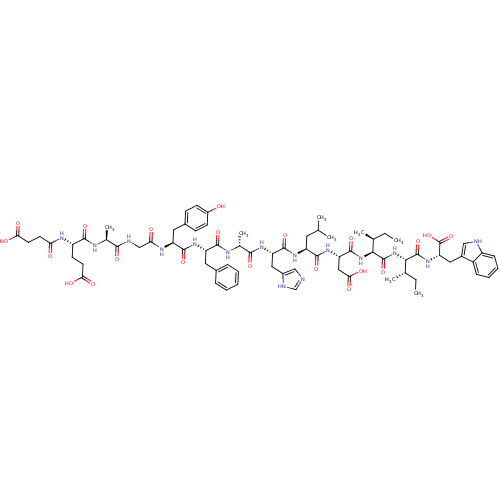 (CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.025 mM i... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.02... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287882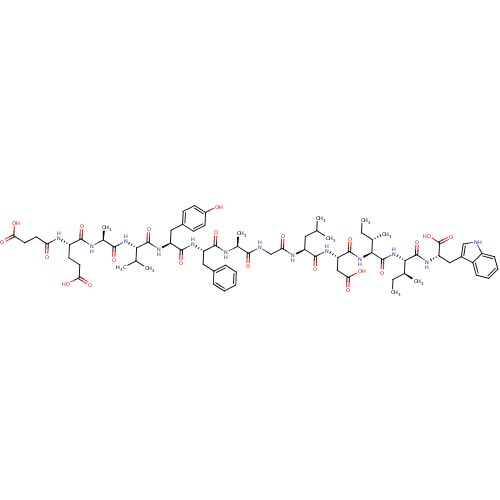 (CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071433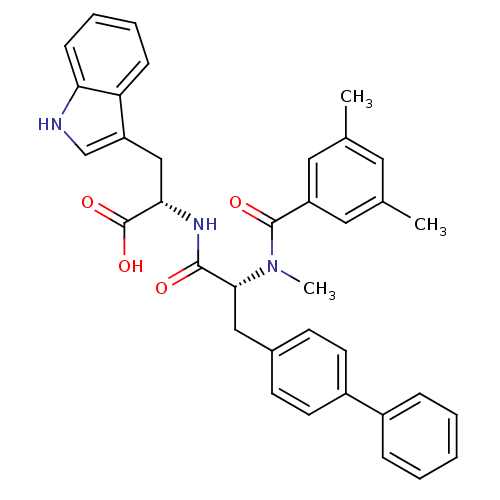 ((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031964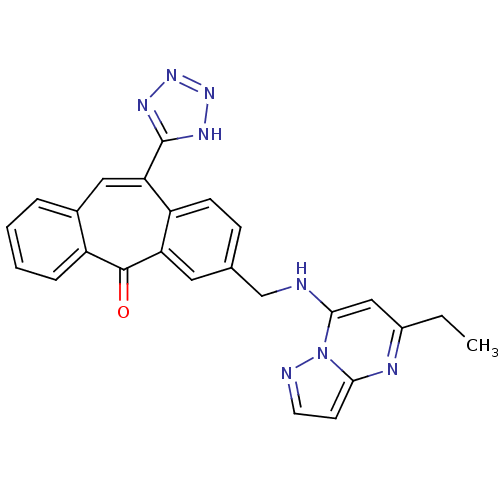 (3-[(5-Ethyl-pyrazolo[1,5-a]pyrimidin-7-ylamino)-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031956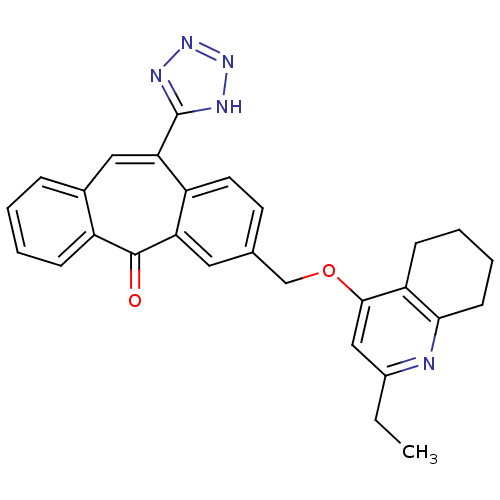 (3-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139906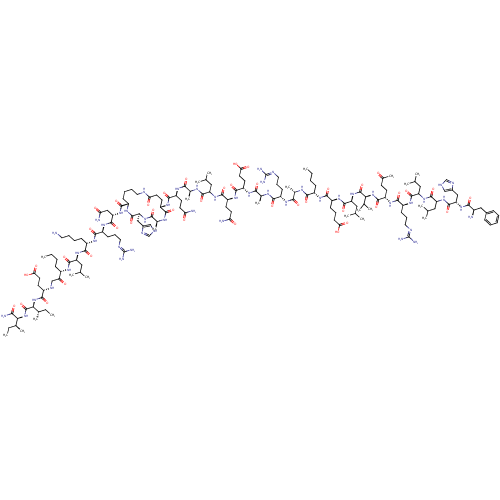 (CHEMBL414991 | DPhe-His-Leu-Leu-Arg-Glu-Val-Leu-Gl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031959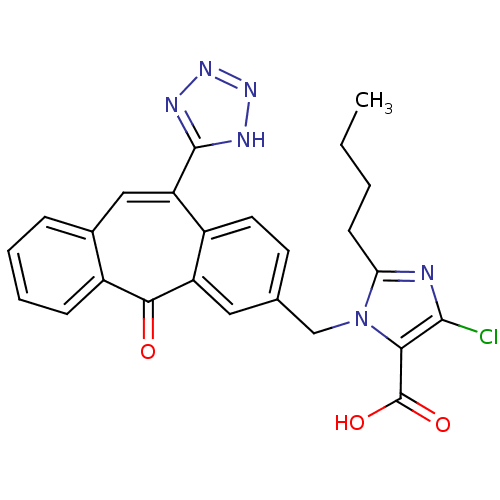 (2-Butyl-5-chloro-3-[5-oxo-11-(1H-tetrazol-5-yl)-5H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705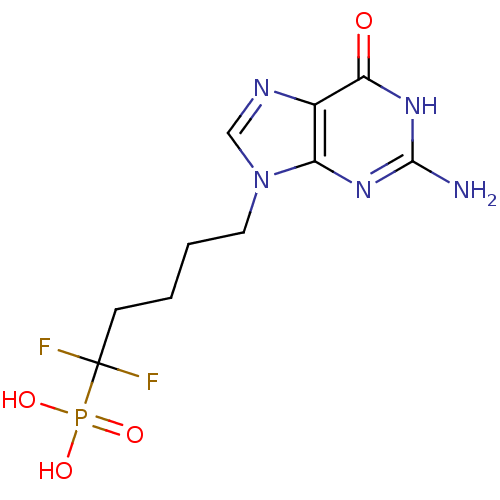 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 0.025 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031965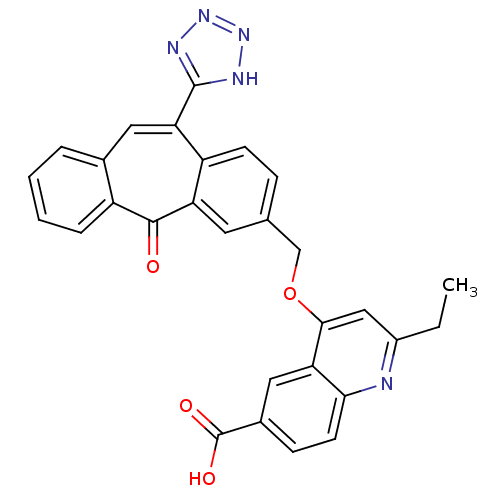 (2-Ethyl-4-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dibenzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031966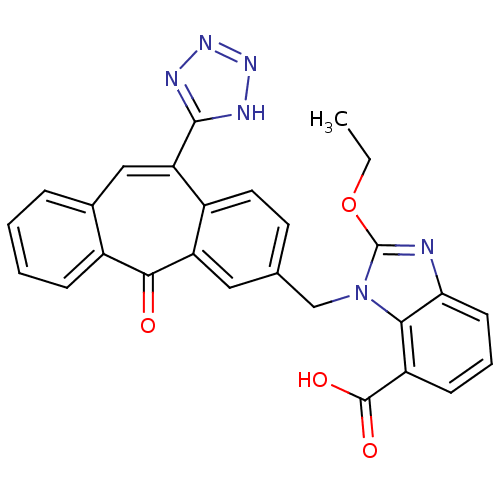 (2-Ethoxy-3-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dibenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 0.025 mM i... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031950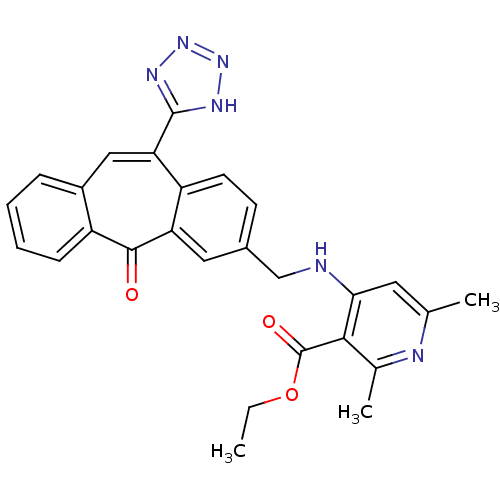 (2,6-Dimethyl-4-{[5-oxo-11-(1H-tetrazol-5-yl)-5H-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078977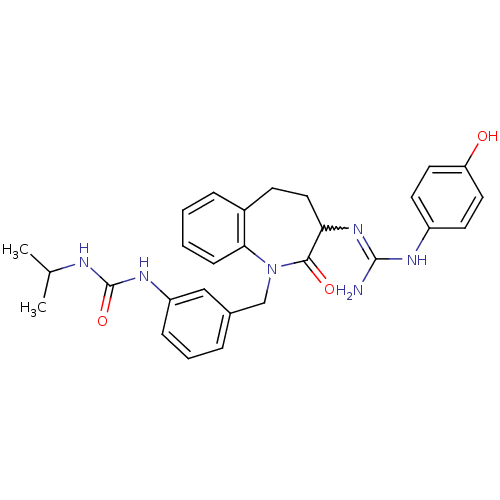 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-guanidino]-2-oxo-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139920 (Ac-(Glu-Aib-Glu-Lys)-Leu-Arg-Lys-Leu-Cha-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139907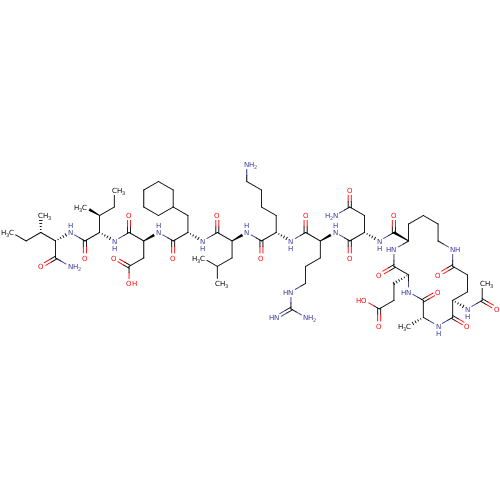 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Cha-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031951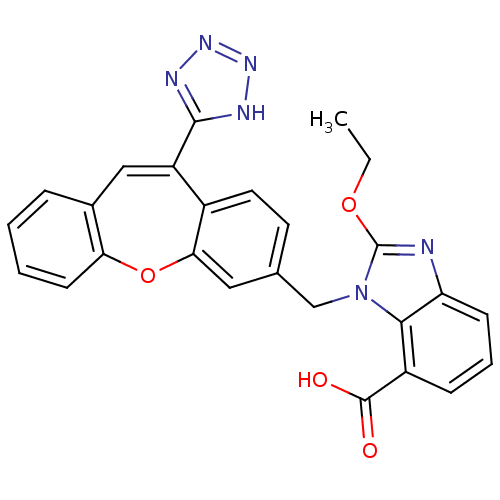 (2-Ethoxy-3-[11-(1H-tetrazol-5-yl)-dibenzo[b,f]oxep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031949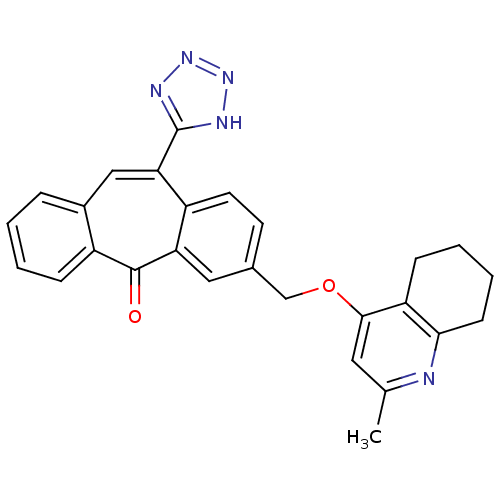 (3-(2-Methyl-5,6,7,8-tetrahydro-quinolin-4-yloxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031963 (2,6-Dimethyl-4-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287878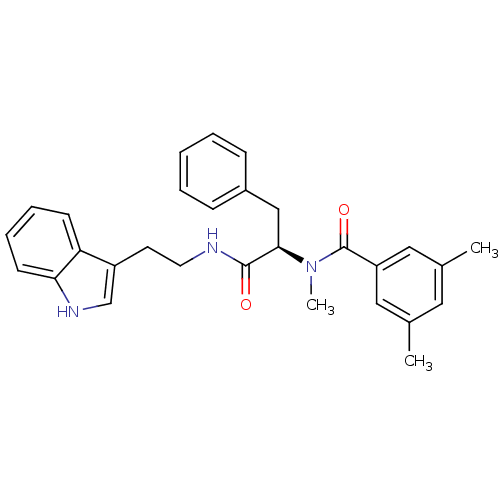 (CGP-49941 | CHEMBL305615 | N-{(R)-1-[2-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078965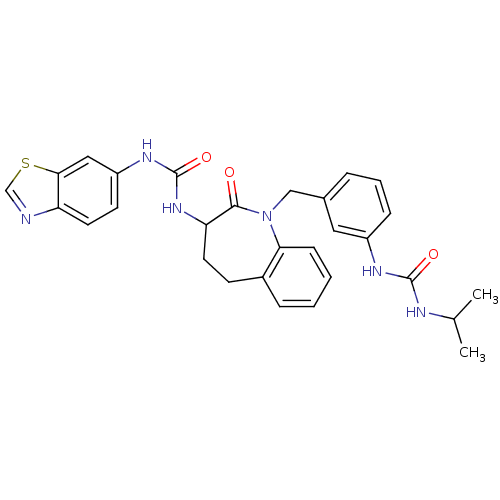 (1-Benzothiazol-6-yl-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50308521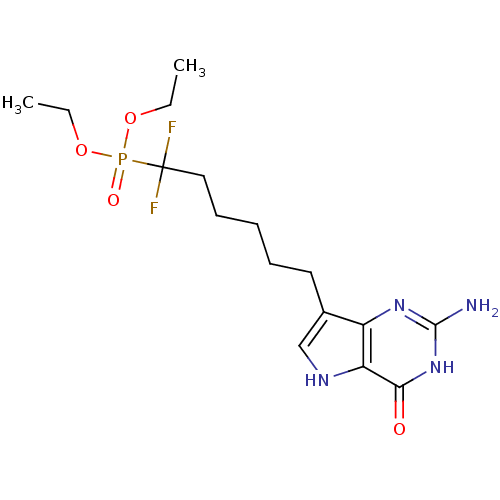 (9-[6',6'-Difluoro-6'-(diethylphosphono)hexyl]-9-de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287888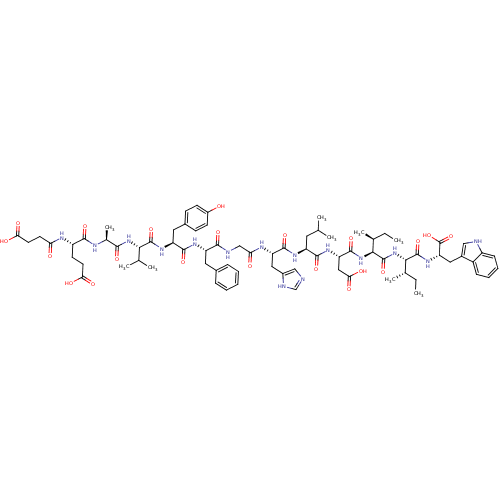 (CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139905 (Ac-(Glu-Ala-Glu-Lys)-Leu-Arg-Lys-Leu-Phe-Asp-Ile-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50308521 (9-[6',6'-Difluoro-6'-(diethylphosphono)hexyl]-9-de...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214708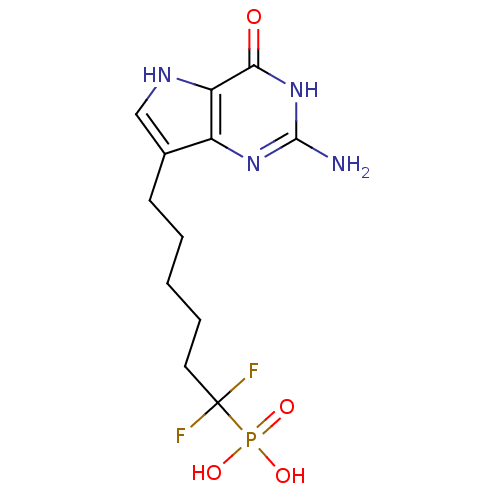 (6-(2-amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf PNP in presence of 1 mM phosphate | Bioorg Med Chem Lett 17: 4173-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.054 BindingDB Entry DOI: 10.7270/Q29P31CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287886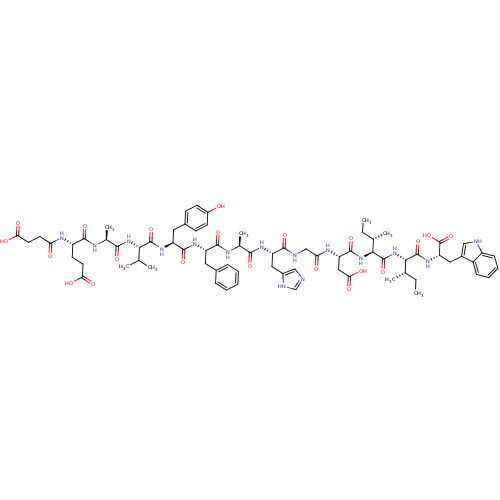 (CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50415479 (CHEMBL601619) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrophotometry in presence of 1 mM inorg... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214707 (9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Inhibition of bovine PNP using 7-methylguanosine as substrate by spectrophotometric based coupled assay | Bioorg Med Chem 20: 6758-69 (2012) Article DOI: 10.1016/j.bmc.2012.08.045 BindingDB Entry DOI: 10.7270/Q23N24H7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of calf spleen PNP assessed as inhibition of 7-methylguanosine phosphorolysis after 50 mins by spectrofluorimetric method in presence of 1... | Bioorg Med Chem 18: 2275-84 (2010) Article DOI: 10.1016/j.bmc.2010.01.062 BindingDB Entry DOI: 10.7270/Q2N58MG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079009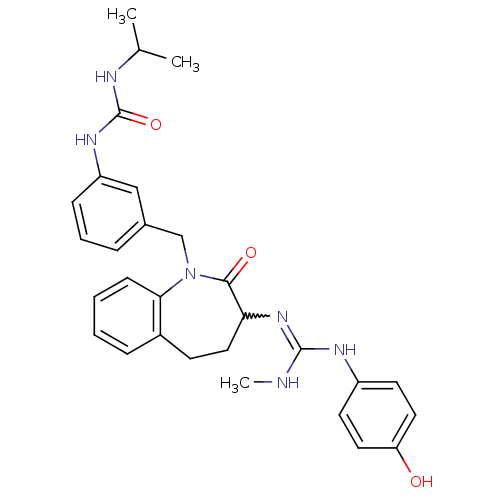 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-N''-methyl-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078970 (1-(2-Fluoro-phenyl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079012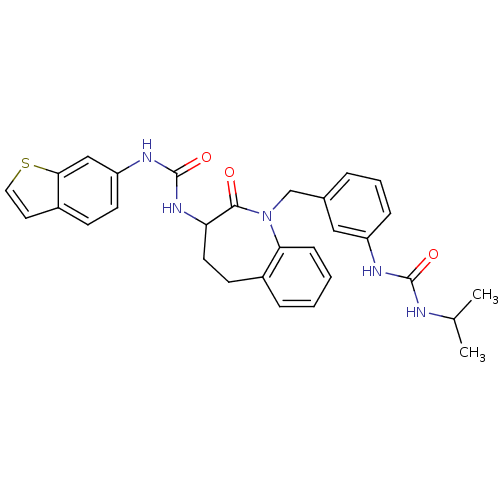 (1-Benzo[b]thiophen-6-yl-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1600 total ) | Next | Last >> |