

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 4 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 4 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 0 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 0 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563426 (CHEMBL4783861) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422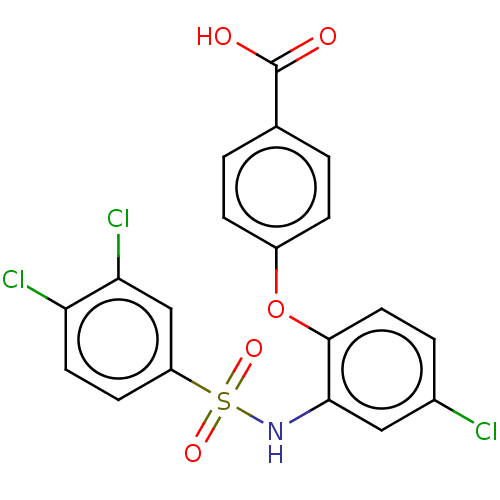 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 0 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 0 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 4 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563422 (CHEMBL4760108) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA-(R) from human CCR2b expressed in human U2OS cell membranes pre-incubated for 4 hrs before radio ligand addition and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179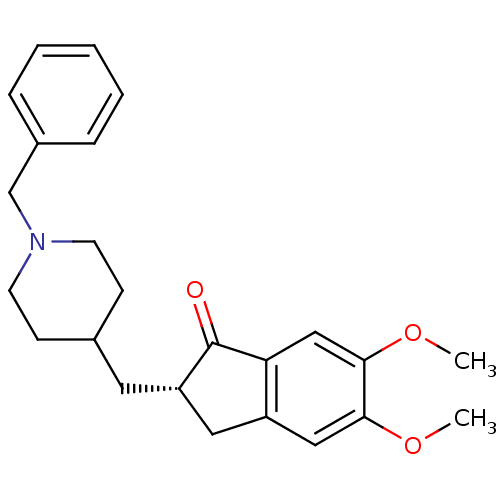 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941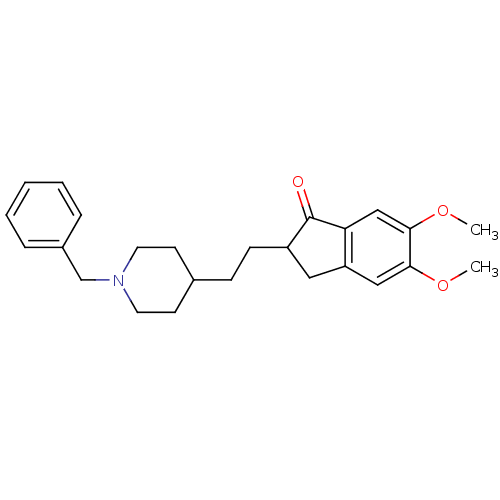 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563424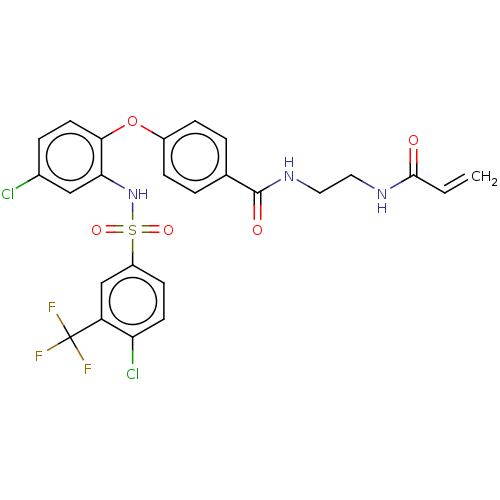 (CHEMBL4793148) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563424 (CHEMBL4793148) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563423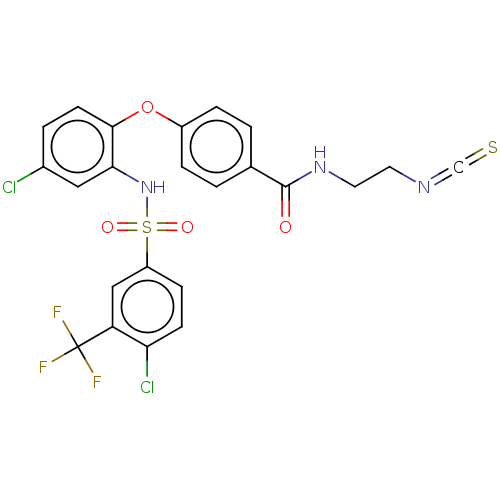 (CHEMBL4791522) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563423 (CHEMBL4791522) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563425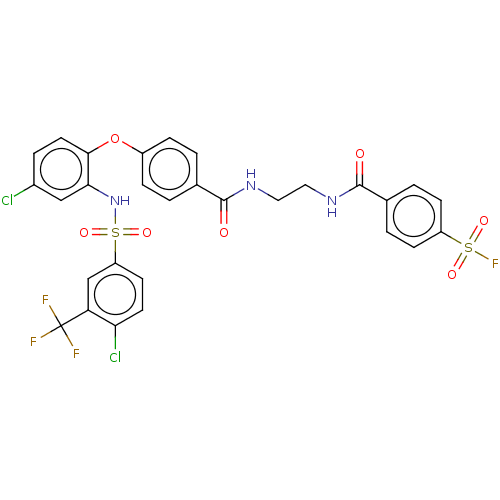 (CHEMBL4795646) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50563425 (CHEMBL4795646) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CCR2-RA from human CCR2b expressed in human U2OS cell membranes incubated for 2 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01137 BindingDB Entry DOI: 10.7270/Q20P13SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104905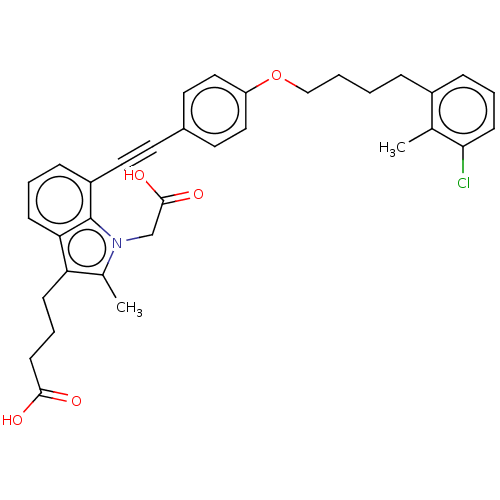 (CHEMBL3597618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50066948 (CHEMBL3403187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104904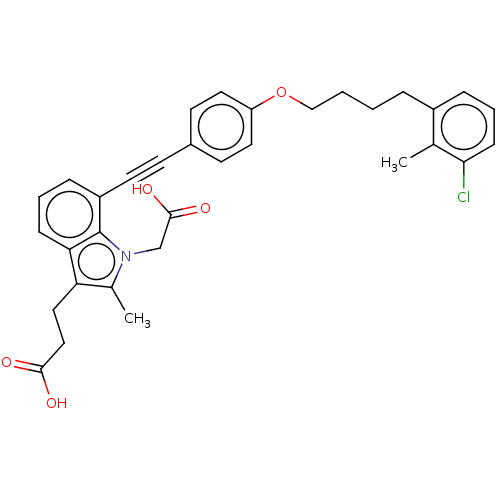 (CHEMBL3597617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104883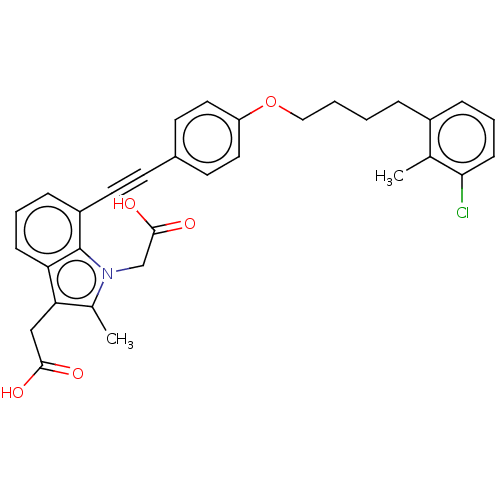 (CHEMBL3597616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50066911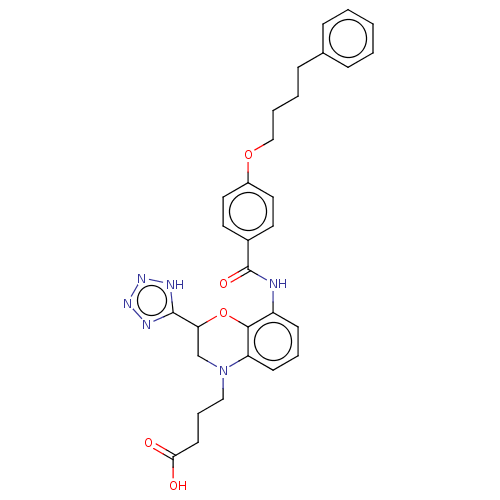 (CHEMBL3401689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104872 (CHEMBL3597535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50066947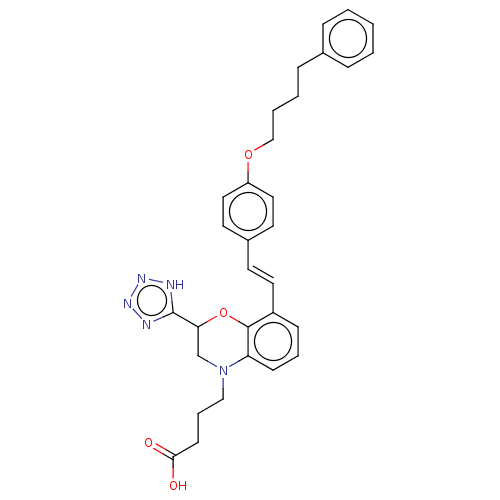 (CHEMBL3403186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 receptor expressed in CHO cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubated ... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104864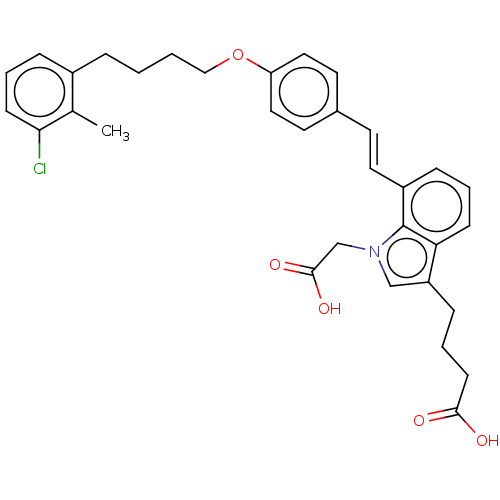 (CHEMBL3597527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104910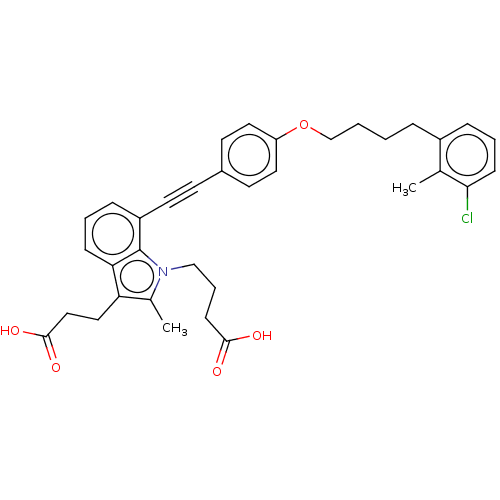 (CHEMBL3597623) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50066911 (CHEMBL3401689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 receptor expressed in HEK293 cells assessed as inhibition of LTD4-inudced intracellular calcium influx preincubat... | Bioorg Med Chem 23: 2079-97 (2015) Article DOI: 10.1016/j.bmc.2015.03.011 BindingDB Entry DOI: 10.7270/Q2SB47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104869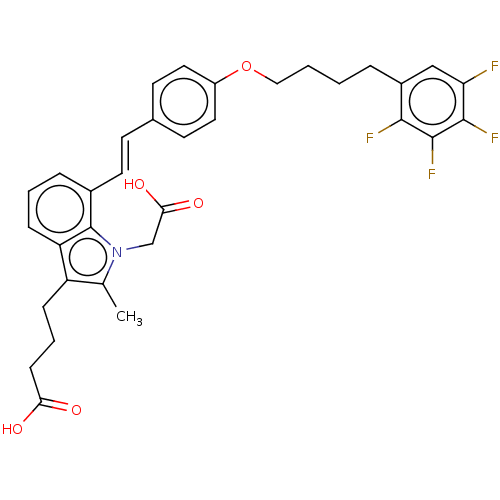 (CHEMBL3597532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104905 (CHEMBL3597618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104883 (CHEMBL3597616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104867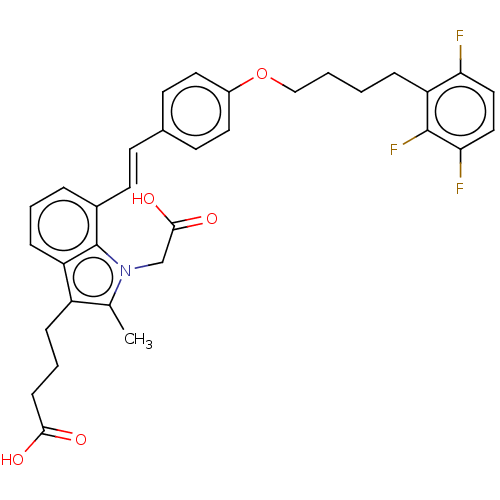 (CHEMBL3597530) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104839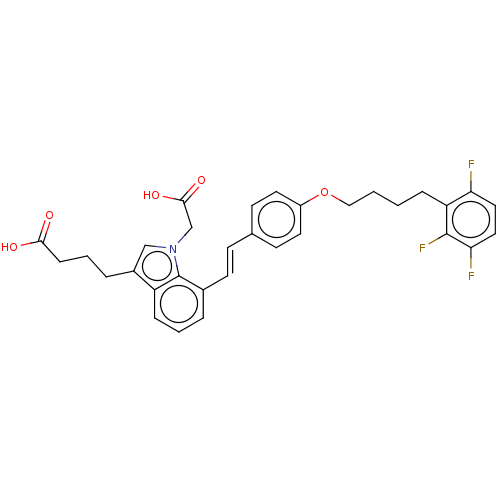 (CHEMBL3597525) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104920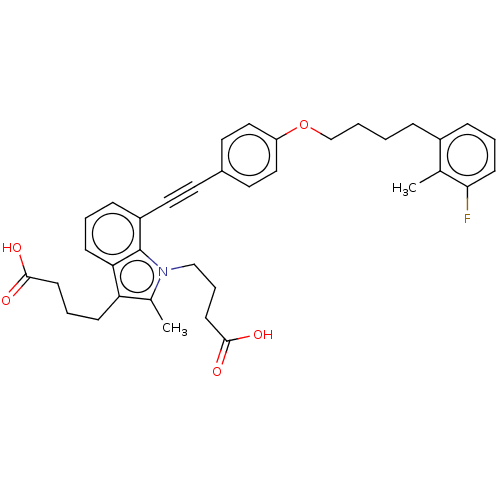 (CHEMBL3597633) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104871 (CHEMBL3597534) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104911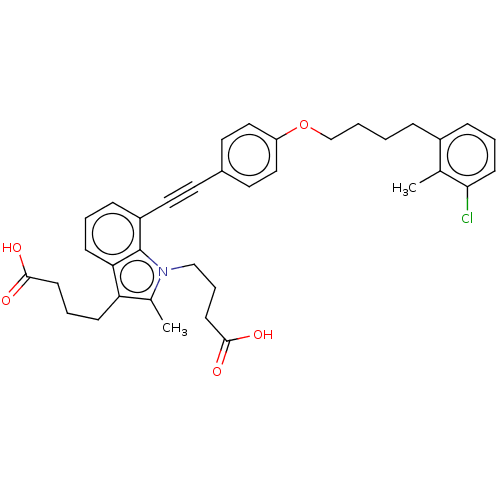 (CHEMBL3597624) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50104836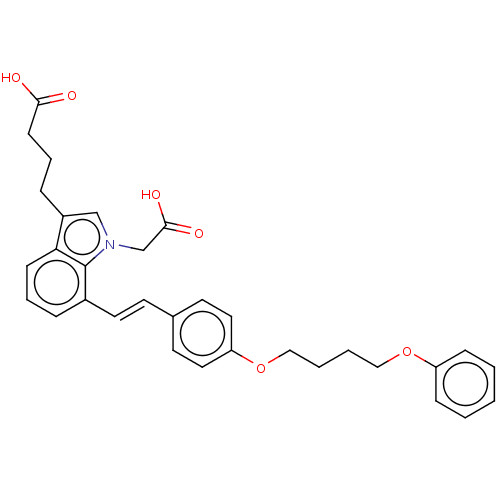 (CHEMBL3597522) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT1 expressed in CHOK1 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pri... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (Homo sapiens (Human)) | BDBM50104869 (CHEMBL3597532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Setsunan University Curated by ChEMBL | Assay Description Antagonist activity at human CysLT2 expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization preincubated for 30 mins pr... | J Med Chem 58: 6093-113 (2015) Article DOI: 10.1021/acs.jmedchem.5b00741 BindingDB Entry DOI: 10.7270/Q2QJ7K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1074 total ) | Next | Last >> |