 Found 146 hits with Last Name = 'irie' and Initial = 'j'
Found 146 hits with Last Name = 'irie' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052
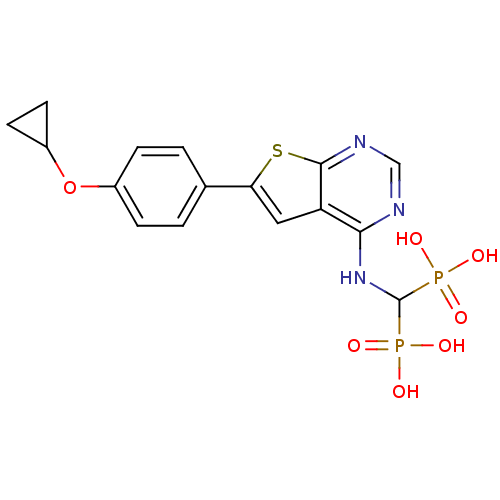
(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094
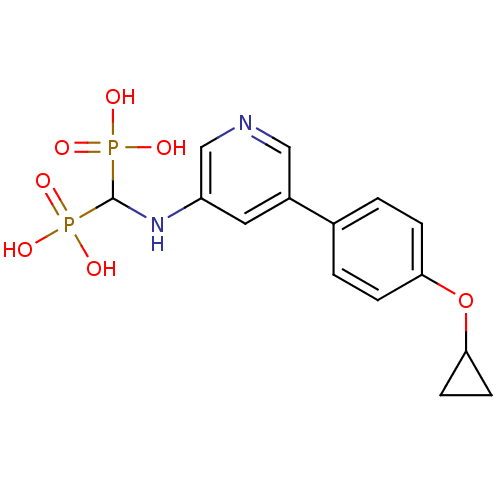
(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115351
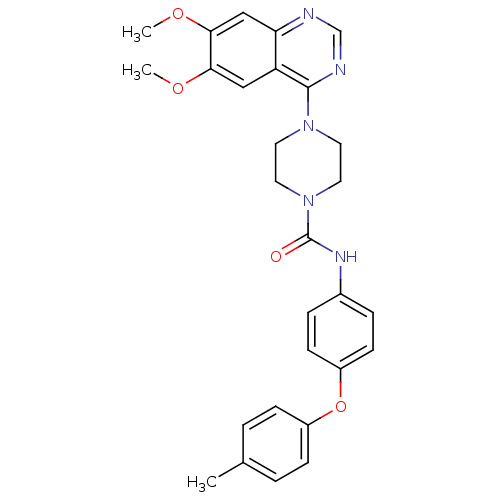
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc(C)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H29N5O4/c1-19-4-8-21(9-5-19)37-22-10-6-20(7-11-22)31-28(34)33-14-12-32(13-15-33)27-23-16-25(35-2)26(36-3)17-24(23)29-18-30-27/h4-11,16-18H,12-15H2,1-3H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115302
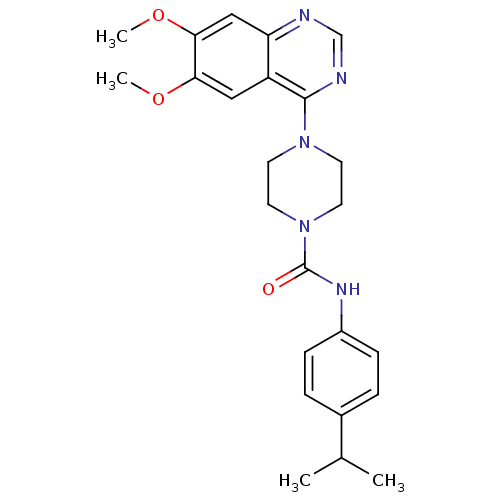
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)C)c2cc1OC Show InChI InChI=1S/C24H29N5O3/c1-16(2)17-5-7-18(8-6-17)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115335
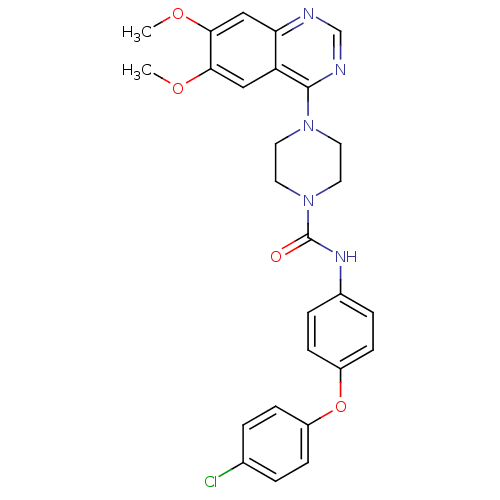
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc(Cl)cc4)cc3)c2cc1OC Show InChI InChI=1S/C27H26ClN5O4/c1-35-24-15-22-23(16-25(24)36-2)29-17-30-26(22)32-11-13-33(14-12-32)27(34)31-19-5-9-21(10-6-19)37-20-7-3-18(28)4-8-20/h3-10,15-17H,11-14H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306
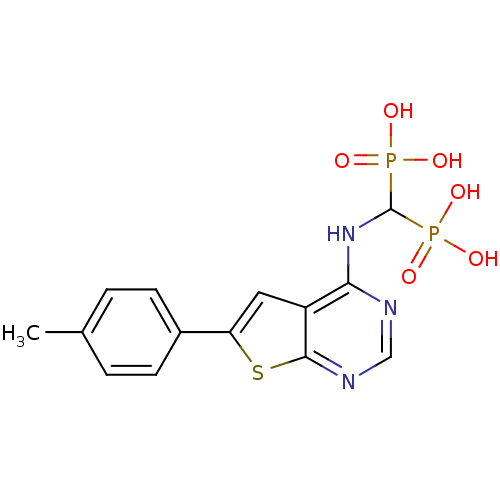
(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115347
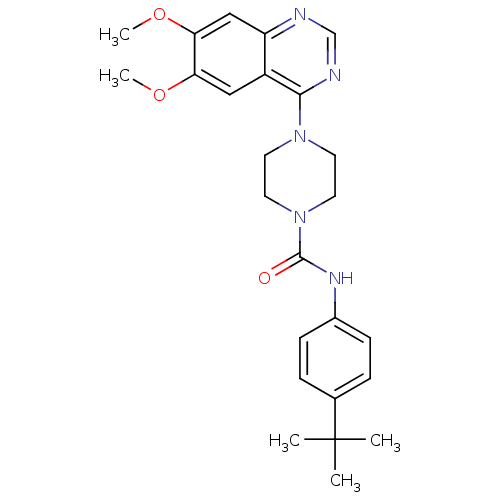
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)(C)C)c2cc1OC Show InChI InChI=1S/C25H31N5O3/c1-25(2,3)17-6-8-18(9-7-17)28-24(31)30-12-10-29(11-13-30)23-19-14-21(32-4)22(33-5)15-20(19)26-16-27-23/h6-9,14-16H,10-13H2,1-5H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138725

((1-phosphono-2-pyridin-3-yl-ethyl)-phosphonic acid...)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)4-6-2-1-3-8-5-6/h1-3,5,7H,4H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50115302

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)C)c2cc1OC Show InChI InChI=1S/C24H29N5O3/c1-16(2)17-5-7-18(8-6-17)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mast/stem cell growth factor receptor (c-Kit kinase) |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50115301
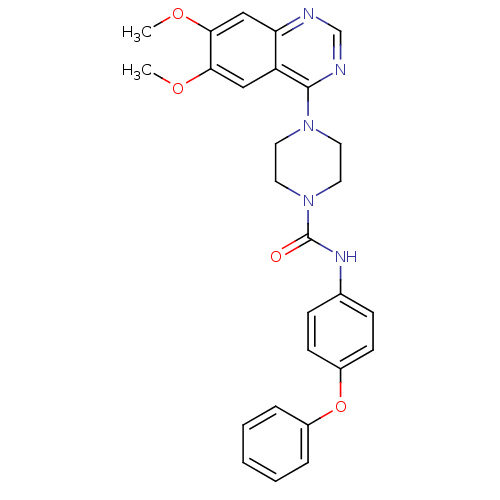
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H27N5O4/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-19-8-10-21(11-9-19)36-20-6-4-3-5-7-20/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mast/stem cell growth factor receptor (c-Kit kinase) |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50115302

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)C)c2cc1OC Show InChI InChI=1S/C24H29N5O3/c1-16(2)17-5-7-18(8-6-17)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 kinase |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50115301

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H27N5O4/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-19-8-10-21(11-9-19)36-20-6-4-3-5-7-20/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor alpha |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115299
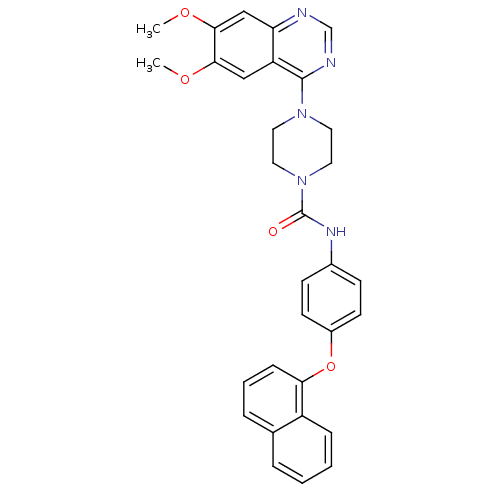
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4cccc5ccccc45)cc3)c2cc1OC Show InChI InChI=1S/C31H29N5O4/c1-38-28-18-25-26(19-29(28)39-2)32-20-33-30(25)35-14-16-36(17-15-35)31(37)34-22-10-12-23(13-11-22)40-27-9-5-7-21-6-3-4-8-24(21)27/h3-13,18-20H,14-17H2,1-2H3,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115331
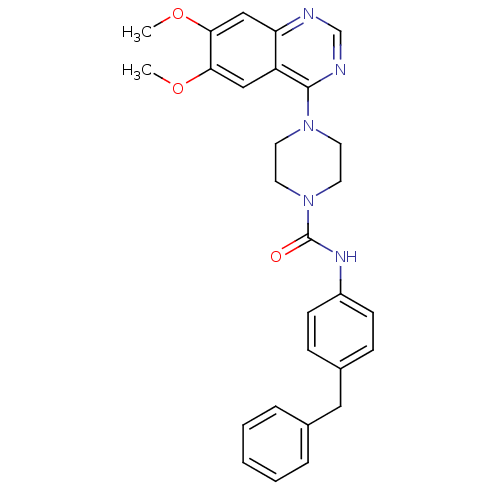
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Cc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C28H29N5O3/c1-35-25-17-23-24(18-26(25)36-2)29-19-30-27(23)32-12-14-33(15-13-32)28(34)31-22-10-8-21(9-11-22)16-20-6-4-3-5-7-20/h3-11,17-19H,12-16H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115302

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)C)c2cc1OC Show InChI InChI=1S/C24H29N5O3/c1-16(2)17-5-7-18(8-6-17)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115319
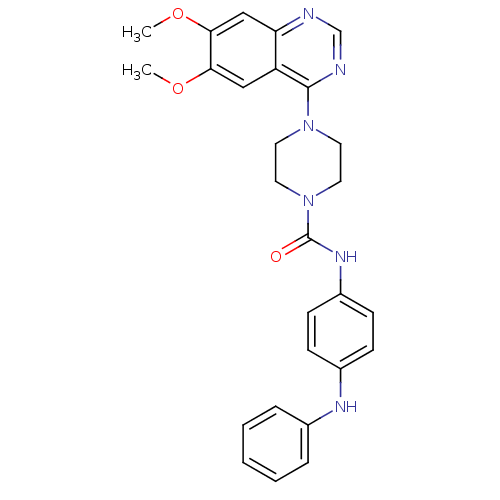
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Nc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H28N6O3/c1-35-24-16-22-23(17-25(24)36-2)28-18-29-26(22)32-12-14-33(15-13-32)27(34)31-21-10-8-20(9-11-21)30-19-6-4-3-5-7-19/h3-11,16-18,30H,12-15H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115301

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H27N5O4/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-19-8-10-21(11-9-19)36-20-6-4-3-5-7-20/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50115302

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)C(C)C)c2cc1OC Show InChI InChI=1S/C24H29N5O3/c1-16(2)17-5-7-18(8-6-17)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor alpha |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115308
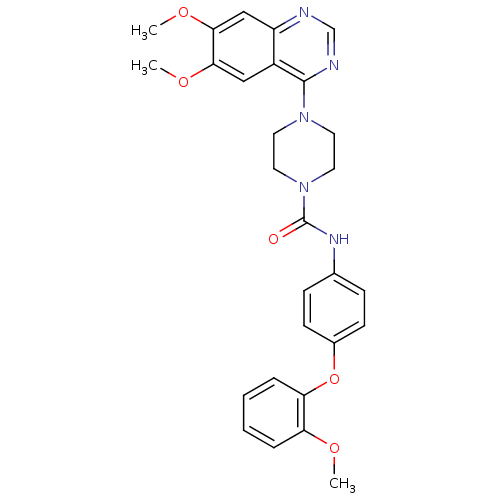
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1ccccc1Oc1ccc(NC(=O)N2CCN(CC2)c2ncnc3cc(OC)c(OC)cc23)cc1 Show InChI InChI=1S/C28H29N5O5/c1-35-23-6-4-5-7-24(23)38-20-10-8-19(9-11-20)31-28(34)33-14-12-32(13-15-33)27-21-16-25(36-2)26(37-3)17-22(21)29-18-30-27/h4-11,16-18H,12-15H2,1-3H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115354
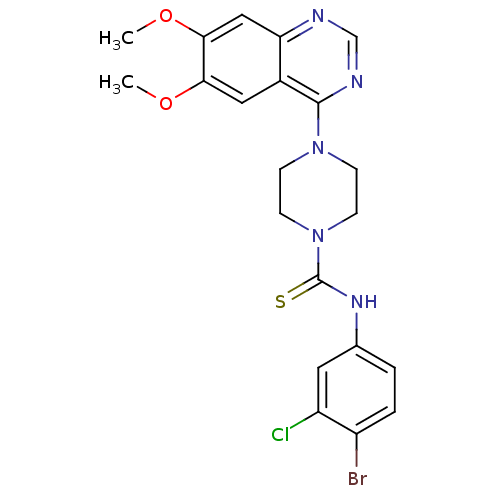
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=S)Nc3ccc(Br)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C21H21BrClN5O2S/c1-29-18-10-14-17(11-19(18)30-2)24-12-25-20(14)27-5-7-28(8-6-27)21(31)26-13-3-4-15(22)16(23)9-13/h3-4,9-12H,5-8H2,1-2H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115306
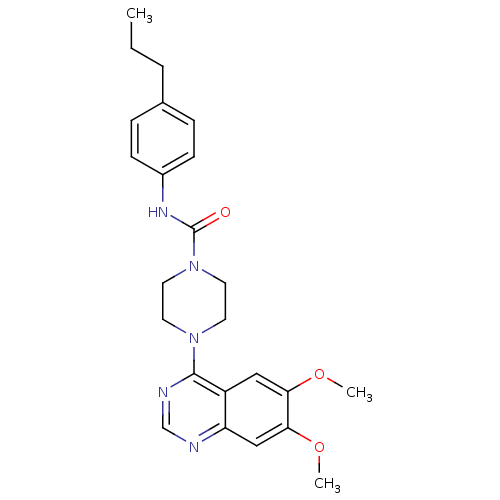
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES CCCc1ccc(NC(=O)N2CCN(CC2)c2ncnc3cc(OC)c(OC)cc23)cc1 Show InChI InChI=1S/C24H29N5O3/c1-4-5-17-6-8-18(9-7-17)27-24(30)29-12-10-28(11-13-29)23-19-14-21(31-2)22(32-3)15-20(19)25-16-26-23/h6-9,14-16H,4-5,10-13H2,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115301

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H27N5O4/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-19-8-10-21(11-9-19)36-20-6-4-3-5-7-20/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50115298
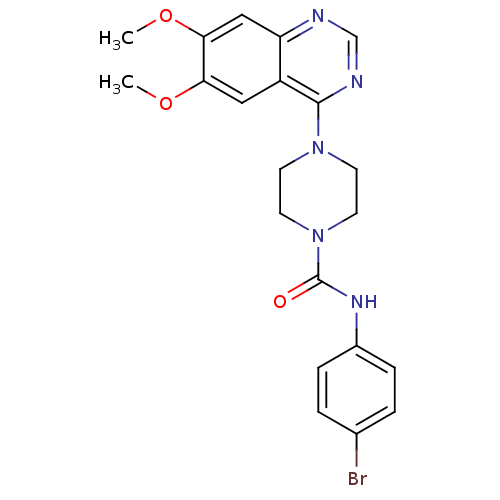
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Br)cc3)c2cc1OC Show InChI InChI=1S/C21H22BrN5O3/c1-29-18-11-16-17(12-19(18)30-2)23-13-24-20(16)26-7-9-27(10-8-26)21(28)25-15-5-3-14(22)4-6-15/h3-6,11-13H,7-10H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mast/stem cell growth factor receptor (c-Kit kinase) |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115327
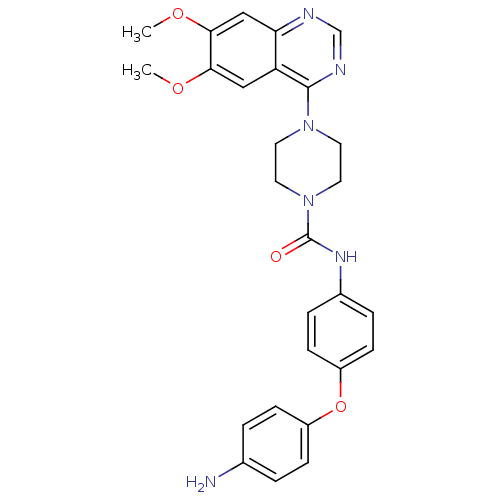
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc(N)cc4)cc3)c2cc1OC Show InChI InChI=1S/C27H28N6O4/c1-35-24-15-22-23(16-25(24)36-2)29-17-30-26(22)32-11-13-33(14-12-32)27(34)31-19-5-9-21(10-6-19)37-20-7-3-18(28)4-8-20/h3-10,15-17H,11-14,28H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115334
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=[SH+])[N-]c3ccc(Cl)c(c3)[N+]([O-])=O)c2cc1OC Show InChI InChI=1S/C21H21ClN6O4S/c1-31-18-10-14-16(11-19(18)32-2)23-12-24-20(14)26-5-7-27(8-6-26)21(33)25-13-3-4-15(22)17(9-13)28(29)30/h3-4,9-12H,5-8H2,1-2H3,(H,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115311
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=[SH+])[N-]c3cccc(c3)[N+]([O-])=O)c2cc1OC Show InChI InChI=1S/C21H22N6O4S/c1-30-18-11-16-17(12-19(18)31-2)22-13-23-20(16)25-6-8-26(9-7-25)21(32)24-14-4-3-5-15(10-14)27(28)29/h3-5,10-13H,6-9H2,1-2H3,(H,24,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022664
(CHEMBL3299048)Show InChI InChI=1S/C15H16N3O3PS/c1-10-2-4-11(5-3-10)13-8-12-14(16-6-7-22(19,20)21)17-9-18-15(12)23-13/h2-5,8-9H,6-7H2,1H3,(H,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022665
(CHEMBL3299049)Show InChI InChI=1S/C15H15N2O3PS/c1-10-2-4-11(5-3-10)14-8-12-13(6-7-21(18,19)20)16-9-17-15(12)22-14/h2-5,8-9H,6-7H2,1H3,(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022666
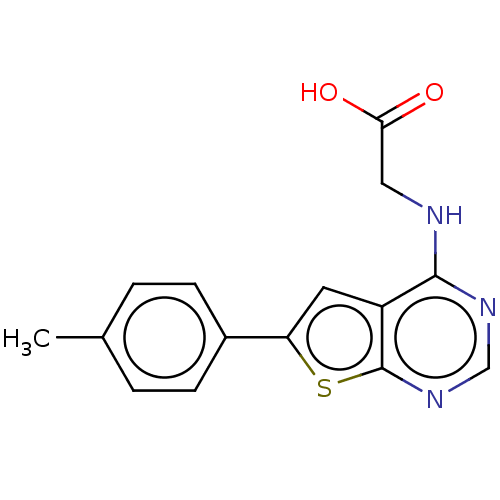
(CHEMBL3299050)Show InChI InChI=1S/C15H13N3O2S/c1-9-2-4-10(5-3-9)12-6-11-14(16-7-13(19)20)17-8-18-15(11)21-12/h2-6,8H,7H2,1H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022667
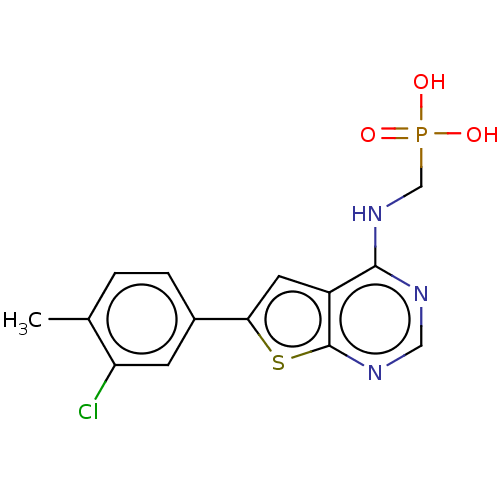
(CHEMBL3299051)Show InChI InChI=1S/C14H13ClN3O3PS/c1-8-2-3-9(4-11(8)15)12-5-10-13(18-7-22(19,20)21)16-6-17-14(10)23-12/h2-6H,7H2,1H3,(H,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022668
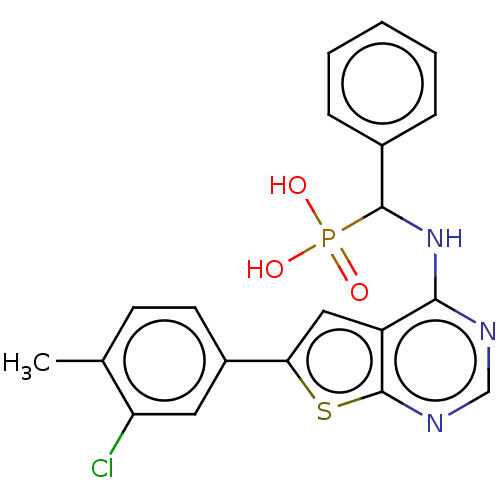
(CHEMBL3299149)Show SMILES Cc1ccc(cc1Cl)-c1cc2c(NC(c3ccccc3)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C20H17ClN3O3PS/c1-12-7-8-14(9-16(12)21)17-10-15-18(22-11-23-20(15)29-17)24-19(28(25,26)27)13-5-3-2-4-6-13/h2-11,19H,1H3,(H,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50022663
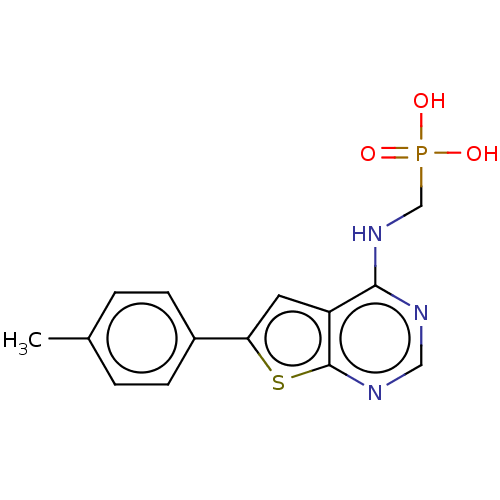
(CHEMBL3299047)Show InChI InChI=1S/C14H14N3O3PS/c1-9-2-4-10(5-3-9)12-6-11-13(17-8-21(18,19)20)15-7-16-14(11)22-12/h2-7H,8H2,1H3,(H,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052

(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094

(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM36510
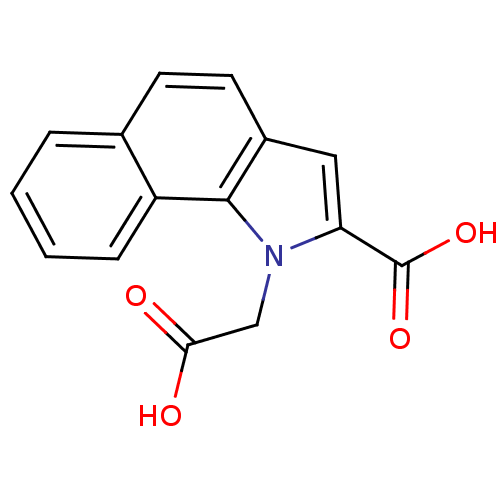
(1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...)Show InChI InChI=1S/C15H11NO4/c17-13(18)8-16-12(15(19)20)7-10-6-5-9-3-1-2-4-11(9)14(10)16/h1-7H,8H2,(H,17,18)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373092
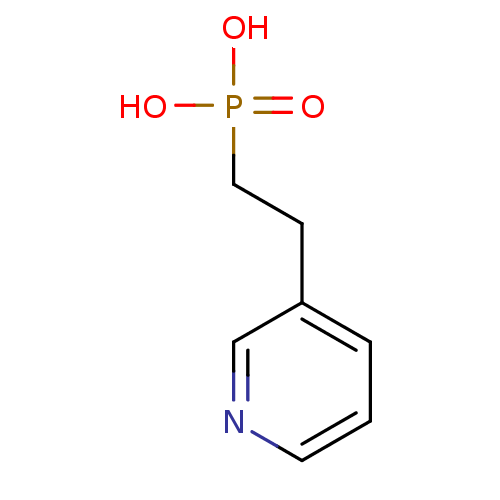
(CHEMBL261311)Show InChI InChI=1S/C7H10NO3P/c9-12(10,11)5-3-7-2-1-4-8-6-7/h1-2,4,6H,3,5H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138725

((1-phosphono-2-pyridin-3-yl-ethyl)-phosphonic acid...)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)4-6-2-1-3-8-5-6/h1-3,5,7H,4H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115344

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES CCCCc1ccc(NC(=O)N2CCN(CC2)c2ncnc3cc(OC)c(OC)cc23)cc1 Show InChI InChI=1S/C25H31N5O3/c1-4-5-6-18-7-9-19(10-8-18)28-25(31)30-13-11-29(12-14-30)24-20-15-22(32-2)23(33-3)16-21(20)26-17-27-24/h7-10,15-17H,4-6,11-14H2,1-3H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115326
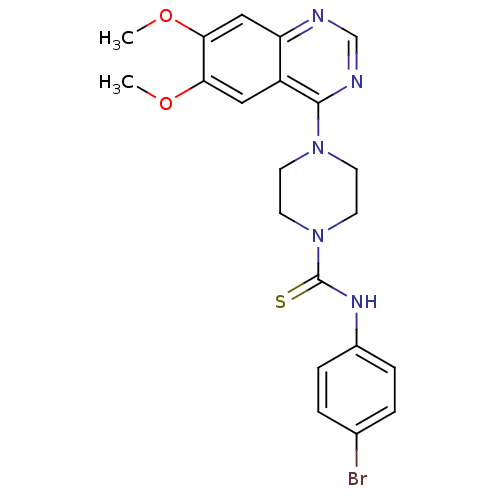
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=S)Nc3ccc(Br)cc3)c2cc1OC Show InChI InChI=1S/C21H22BrN5O2S/c1-28-18-11-16-17(12-19(18)29-2)23-13-24-20(16)26-7-9-27(10-8-26)21(30)25-15-5-3-14(22)4-6-15/h3-6,11-13H,7-10H2,1-2H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115350
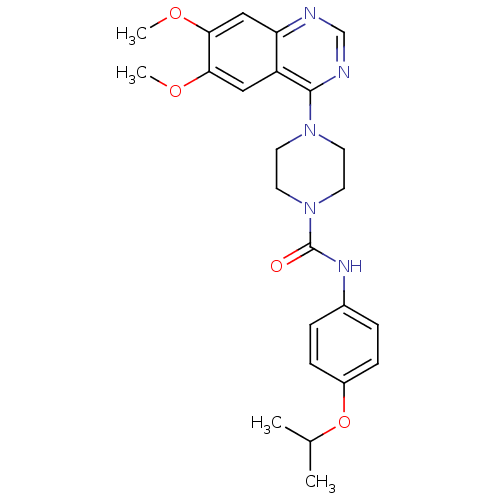
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(OC(C)C)cc3)c2cc1OC Show InChI InChI=1S/C24H29N5O4/c1-16(2)33-18-7-5-17(6-8-18)27-24(30)29-11-9-28(10-12-29)23-19-13-21(31-3)22(32-4)14-20(19)25-15-26-23/h5-8,13-16H,9-12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50115301

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C27H27N5O4/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-19-8-10-21(11-9-19)36-20-6-4-3-5-7-20/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 kinase |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115295
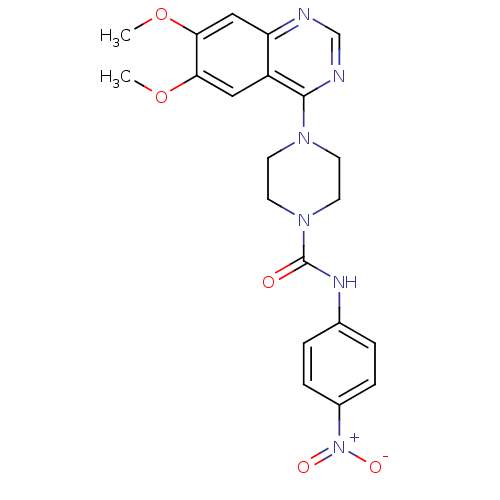
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)[N+]([O-])=O)c2cc1OC Show InChI InChI=1S/C21H22N6O5/c1-31-18-11-16-17(12-19(18)32-2)22-13-23-20(16)25-7-9-26(10-8-25)21(28)24-14-3-5-15(6-4-14)27(29)30/h3-6,11-13H,7-10H2,1-2H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115337
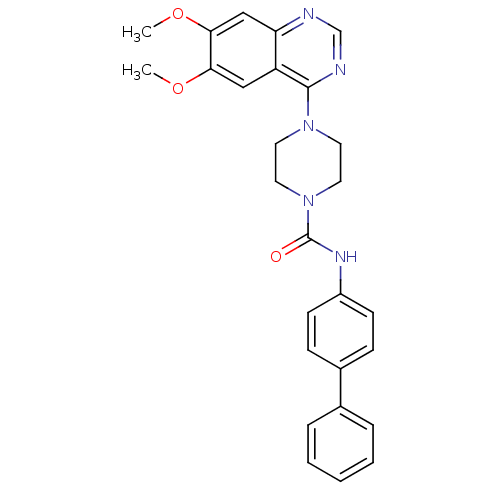
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(cc3)-c3ccccc3)c2cc1OC Show InChI InChI=1S/C27H27N5O3/c1-34-24-16-22-23(17-25(24)35-2)28-18-29-26(22)31-12-14-32(15-13-31)27(33)30-21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,16-18H,12-15H2,1-2H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115298

(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Br)cc3)c2cc1OC Show InChI InChI=1S/C21H22BrN5O3/c1-29-18-11-16-17(12-19(18)30-2)23-13-24-20(16)26-7-9-27(10-8-26)21(28)25-15-5-3-14(22)4-6-15/h3-6,11-13H,7-10H2,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50115338
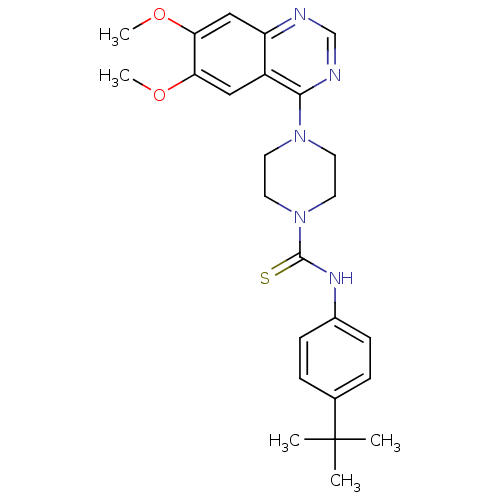
(4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-car...)Show SMILES COc1cc2ncnc(N3CCN(CC3)C(=S)Nc3ccc(cc3)C(C)(C)C)c2cc1OC Show InChI InChI=1S/C25H31N5O2S/c1-25(2,3)17-6-8-18(9-7-17)28-24(33)30-12-10-29(11-13-30)23-19-14-21(31-4)22(32-5)15-20(19)26-16-27-23/h6-9,14-16H,10-13H2,1-5H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Platelet-derived growth factor receptor beta phosphorylation |
J Med Chem 45: 3057-66 (2002)
BindingDB Entry DOI: 10.7270/Q2RX9BDP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data