

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365698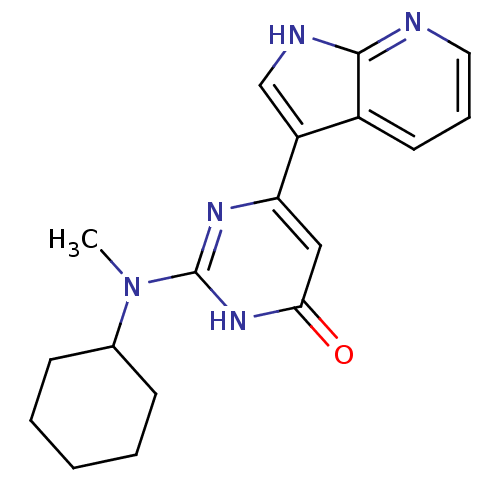 (CHEMBL1958411) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365703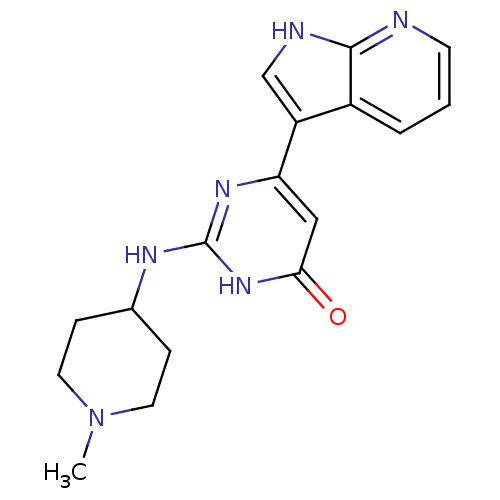 (CHEMBL1958417) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365699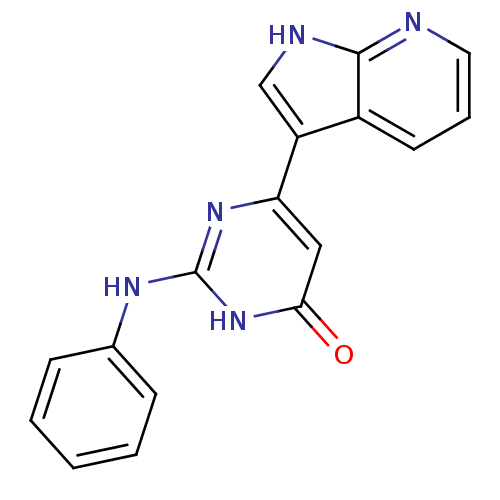 (CHEMBL1958412) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365702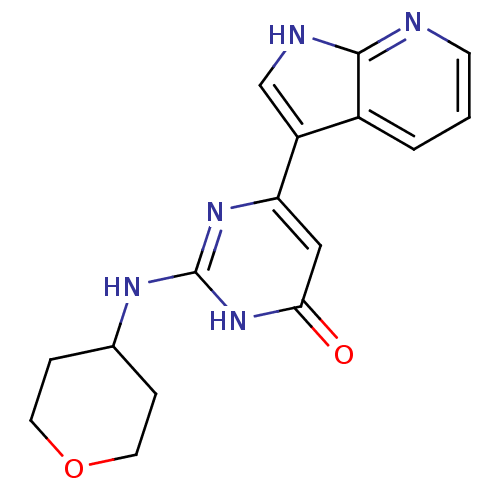 (CHEMBL1958416) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15131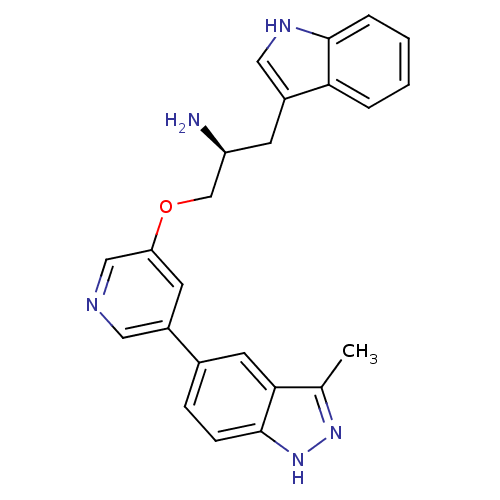 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365700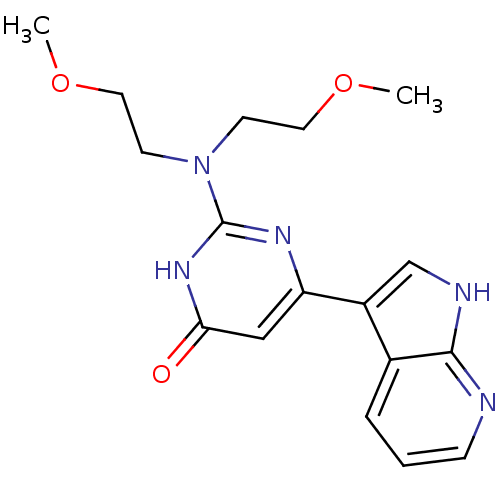 (CHEMBL1958414) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365701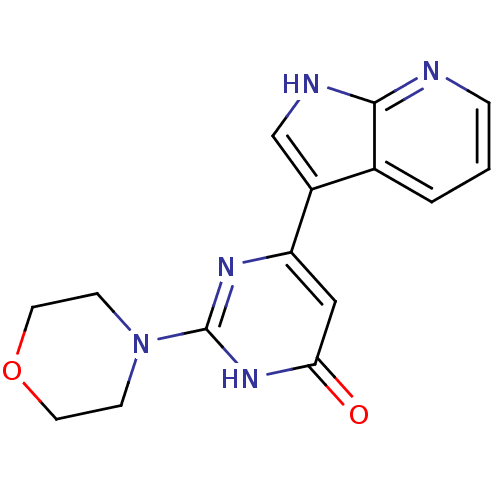 (CHEMBL1958415) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365706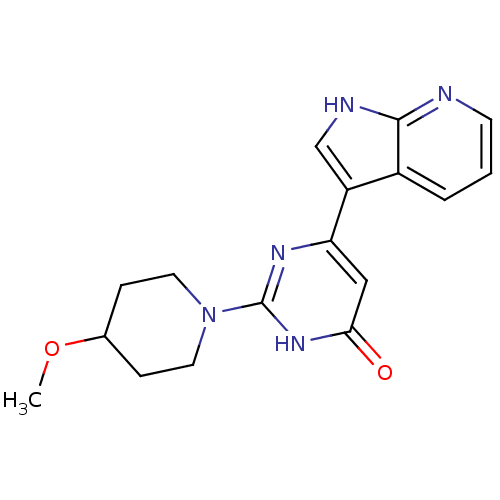 (CHEMBL1958420) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522512 (CHEMBL4544916) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522506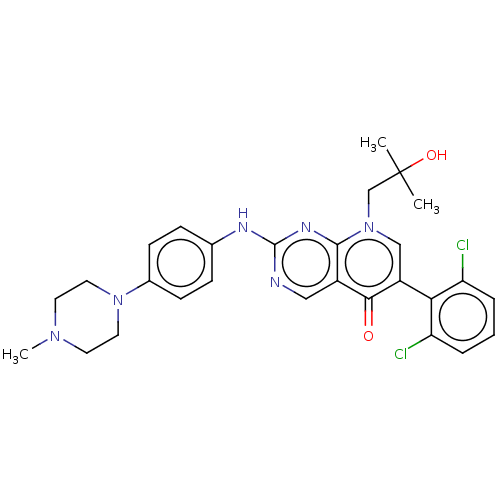 (CHEMBL4562919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522503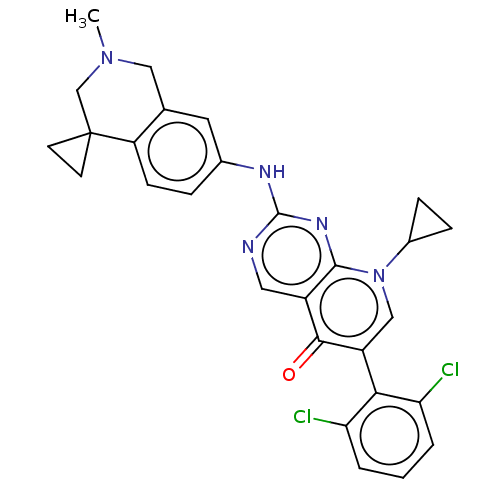 (CHEMBL4454675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365705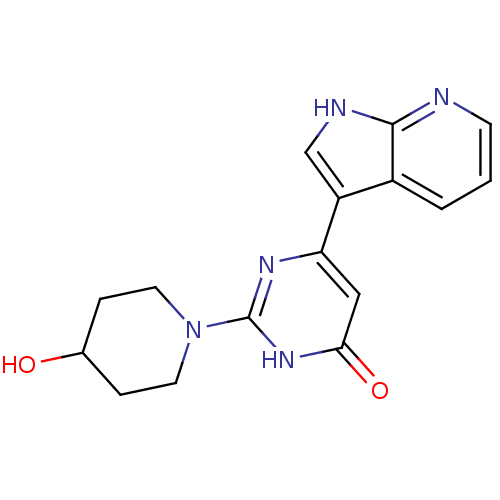 (CHEMBL1958419) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522508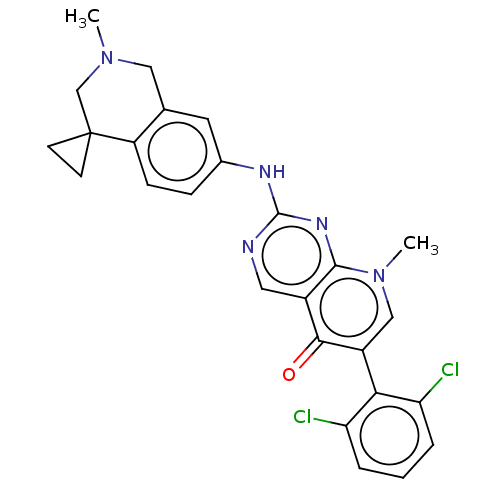 (CHEMBL4441166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365704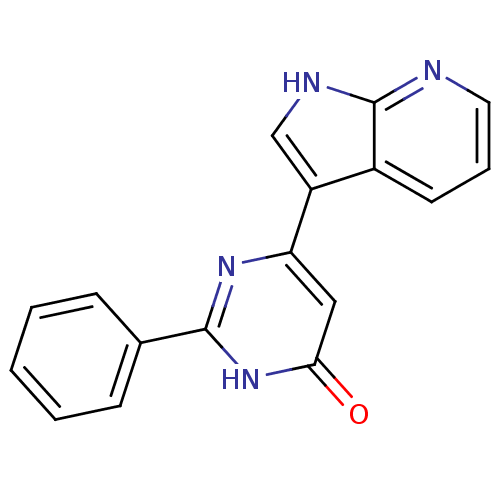 (CHEMBL1958418) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50365695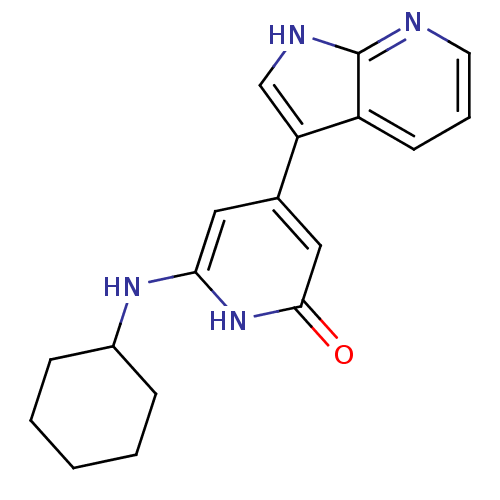 (CHEMBL1958408) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human Cdc7 using biotin-C6linker-TPSDSLIYDDGLS as substrate after 1 hr | ACS Med Chem Lett 4: 211-5 (2013) Article DOI: 10.1021/ml300348c BindingDB Entry DOI: 10.7270/Q2DR2WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522514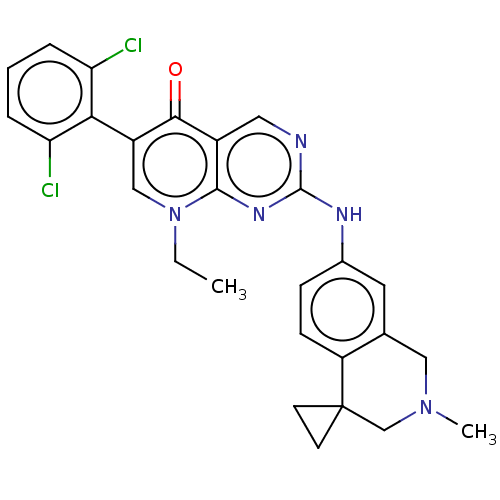 (CHEMBL4554796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522509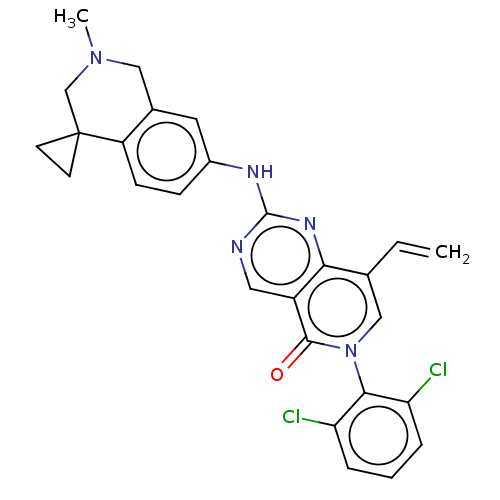 (CHEMBL4444364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522511 (CHEMBL4443172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522502 (CHEMBL4529353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365693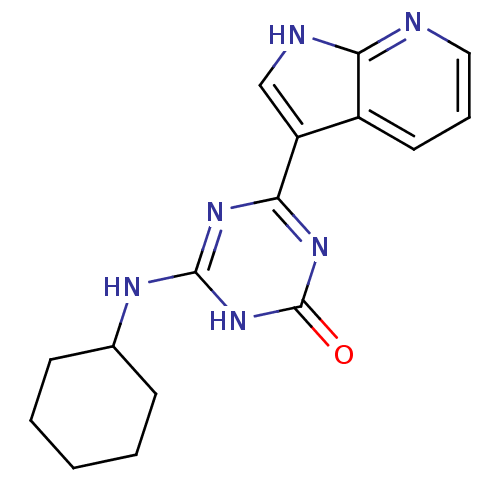 (CHEMBL1958406) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by PDSP Ki Database | J Med Chem 40: 2706-25 (1997) Article DOI: 10.1021/jm970265x BindingDB Entry DOI: 10.7270/Q27M06F9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387945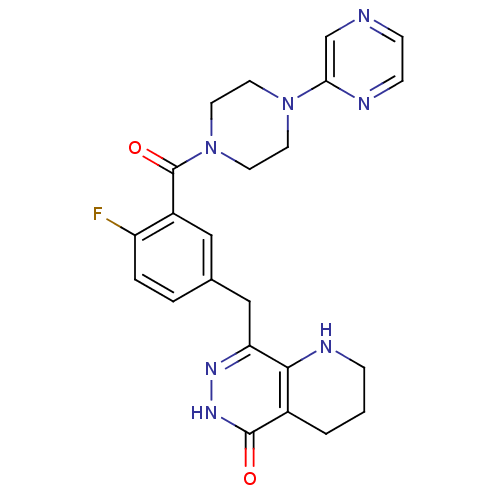 (CHEMBL2058927 | US9283222, 511) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185854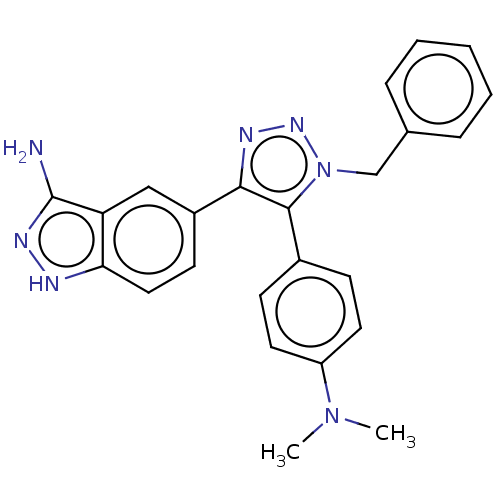 (US9163007, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM35048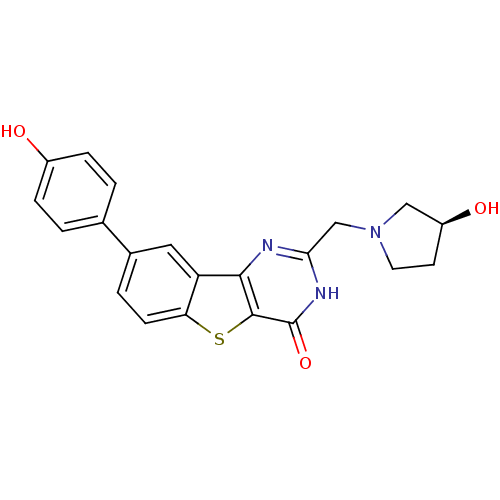 (benzothienopyrimidinone deriv., 20c) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50388878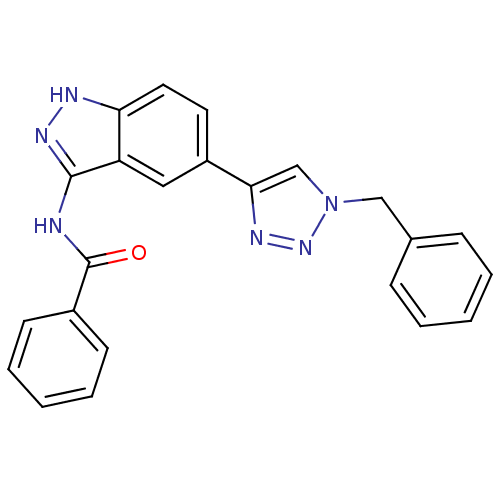 (CHEMBL1999931 | US9163007, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387946 (CHEMBL2058928 | US9283222, 536) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185901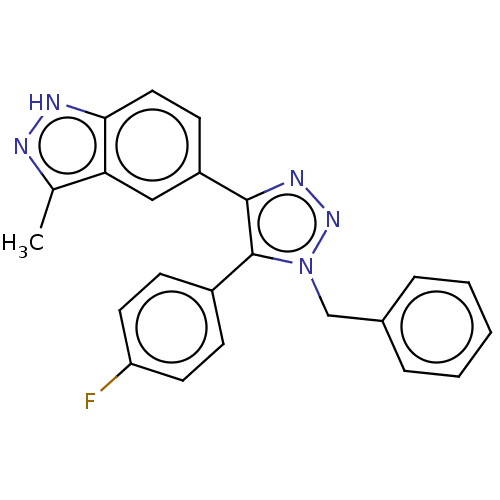 (US9163007, 408) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.428 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185899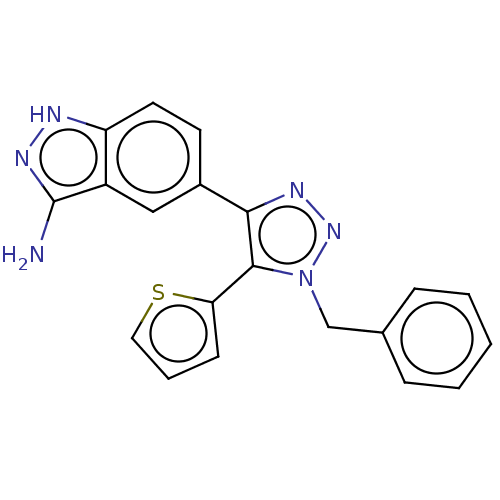 (US9163007, 406) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185888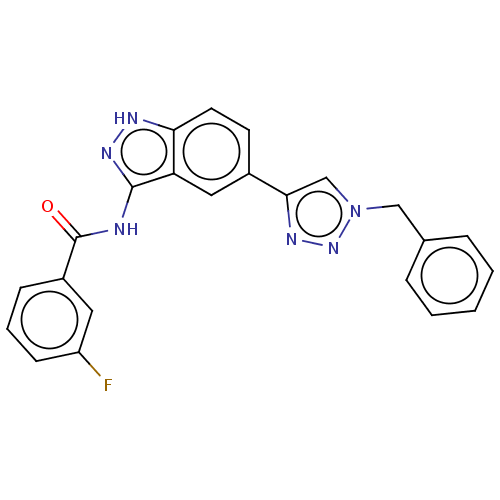 (US9163007, 395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522513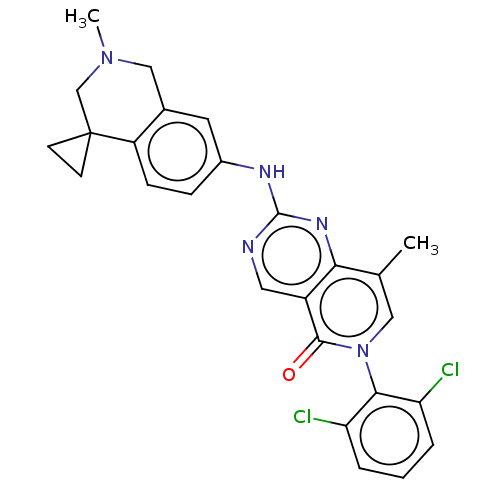 (CHEMBL4526423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365692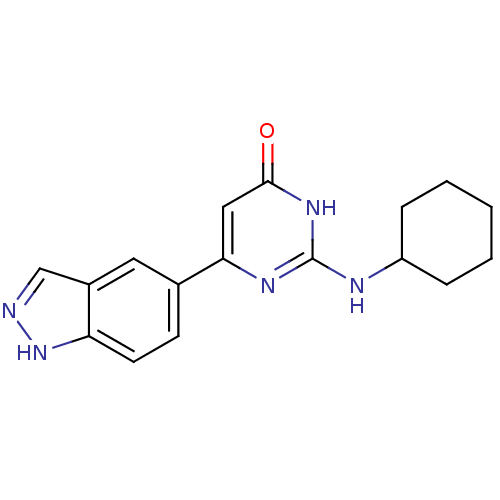 (CHEMBL1958404) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM50365695 (CHEMBL1958408) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human CDC7/DBF4 expressed using baculovirus expression system assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by ... | Bioorg Med Chem Lett 22: 1940-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.041 BindingDB Entry DOI: 10.7270/Q2DR2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522507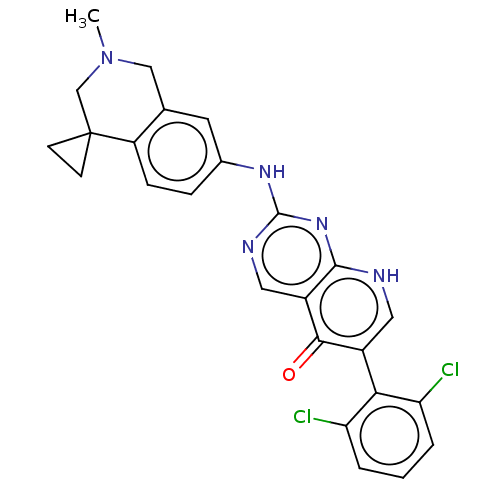 (CHEMBL4470852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522510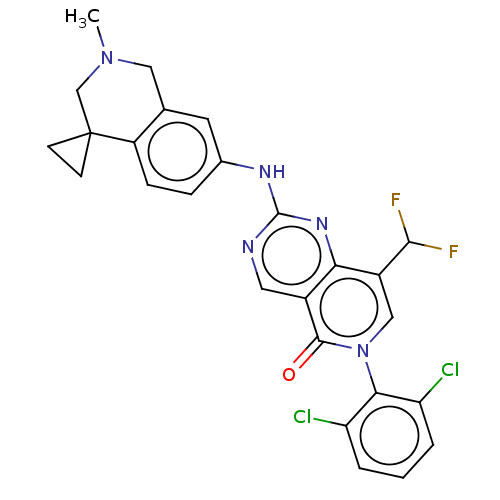 (CHEMBL4447769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM35042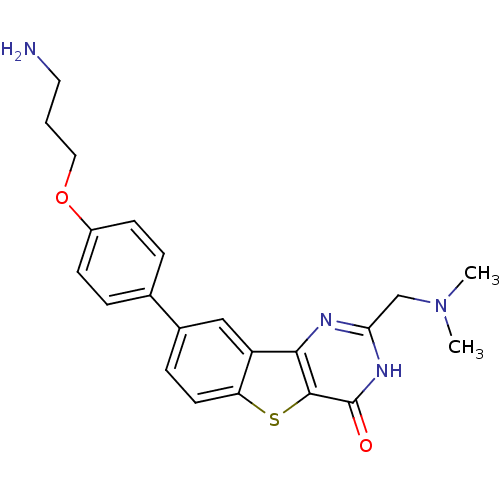 (benzothienopyrimidinone deriv., 18b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50388878 (CHEMBL1999931 | US9163007, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185839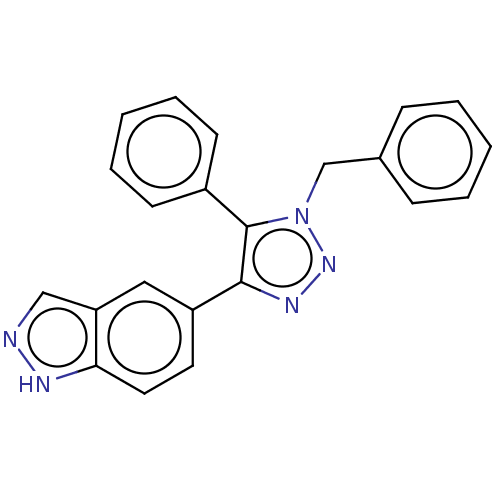 (US9163007, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.601 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387914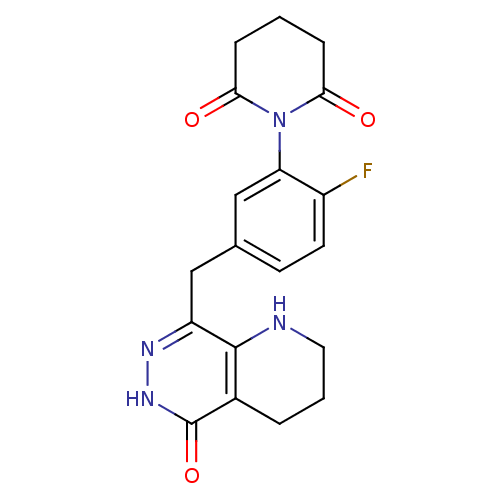 (CHEMBL2058682 | US9283222, 507) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM35048 (benzothienopyrimidinone deriv., 20c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387941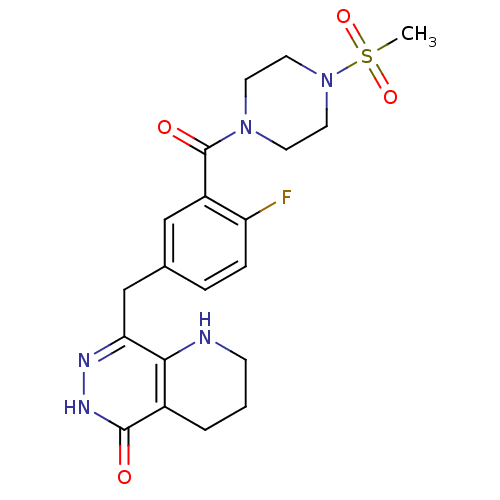 (CHEMBL2058922 | US9283222, 556) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM16300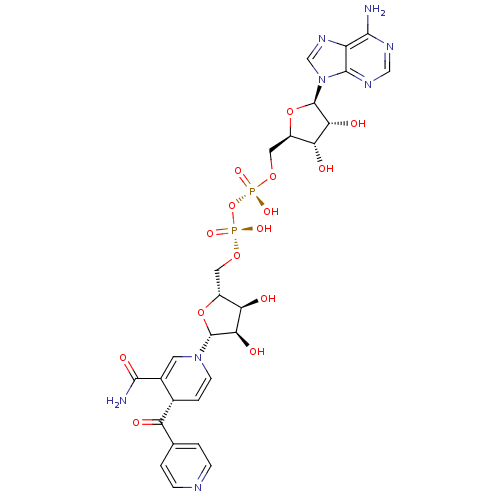 (INH-NAD Adduct | ISONICOTINIC-ACETYL-NICOTINAMIDE-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.75 | -51.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 22 |
SUNY Stony Brook | Assay Description Inhibition constants (Ki) were calculated by determining the kcat and Km (DDCoA) values at several fixed inhibitor concentrations. The inhibition dat... | ACS Chem Biol 1: 43-53 (2006) Article DOI: 10.1021/cb0500042 BindingDB Entry DOI: 10.7270/Q2K35RWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50300018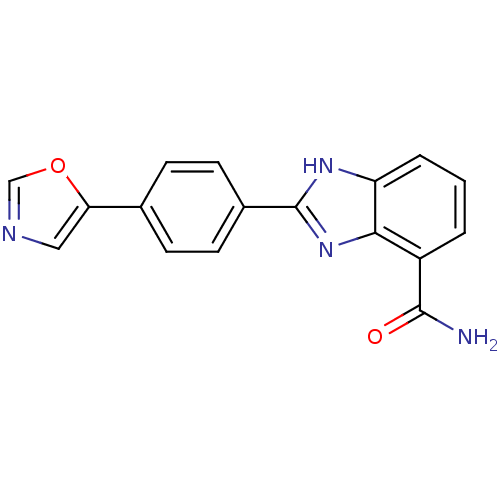 (2-(4-(Oxazol-5-yl)phenyl)-1H-benzo[d]imidazole-4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 by scintillation counting | J Med Chem 52: 6803-13 (2009) Article DOI: 10.1021/jm900697r BindingDB Entry DOI: 10.7270/Q2DN4548 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185858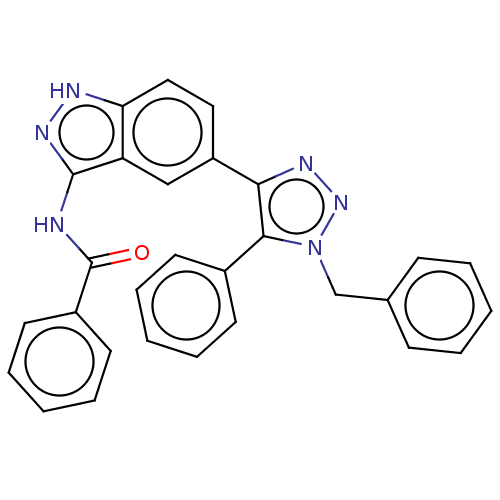 (US9163007, 198) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM35043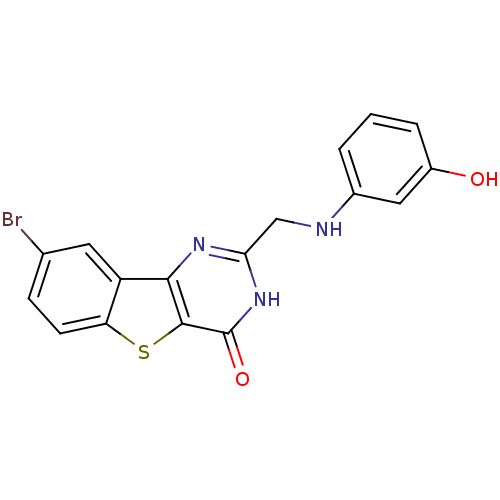 (benzothienopyrimidinone deriv., 19a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185846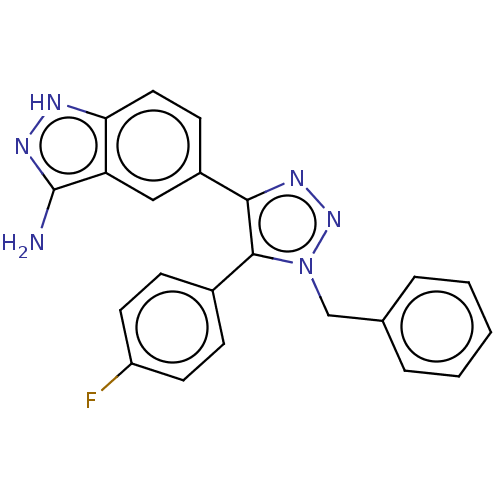 (US9163007, 151) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM35050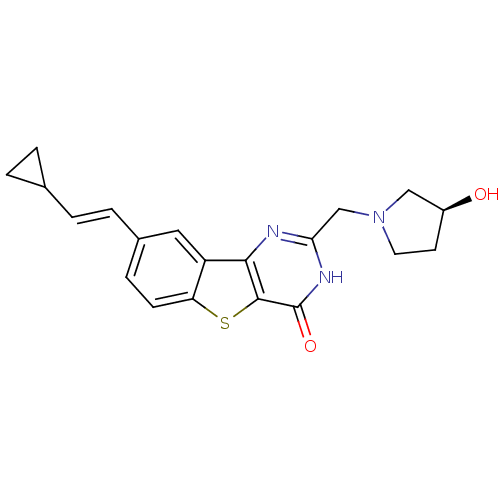 (benzothienopyrimidinone deriv., 20e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387944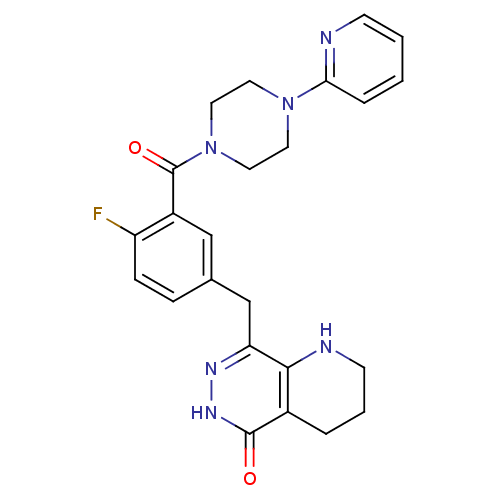 (CHEMBL2058926 | US9283222, 545) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387948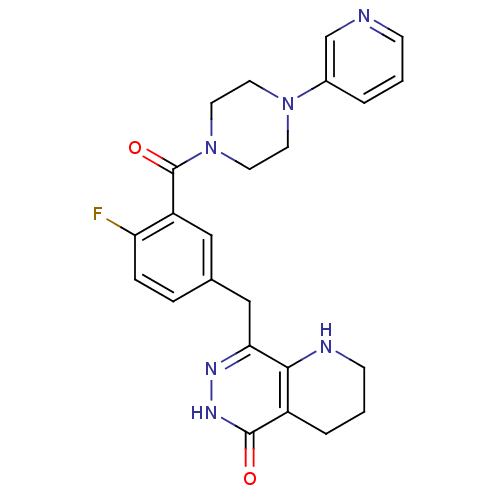 (CHEMBL2058925 | US9283222, 695) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting | Bioorg Med Chem 20: 4635-45 (2012) Article DOI: 10.1016/j.bmc.2012.06.021 BindingDB Entry DOI: 10.7270/Q2PN96P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM35050 (benzothienopyrimidinone deriv., 20e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50426461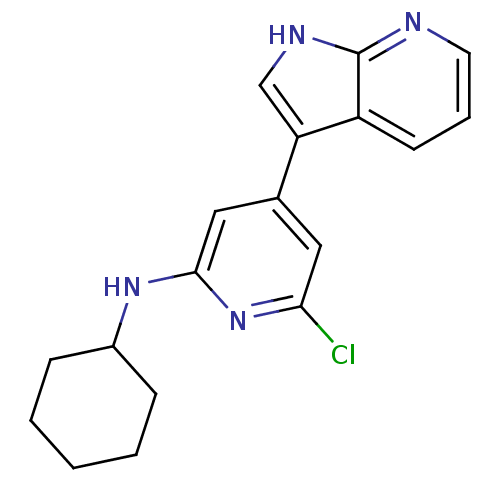 (CHEMBL2322677) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human Cdc7 using biotin-C6linker-TPSDSLIYDDGLS as substrate after 1 hr | ACS Med Chem Lett 4: 211-5 (2013) Article DOI: 10.1021/ml300348c BindingDB Entry DOI: 10.7270/Q2DR2WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3017 total ) | Next | Last >> |