

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50079577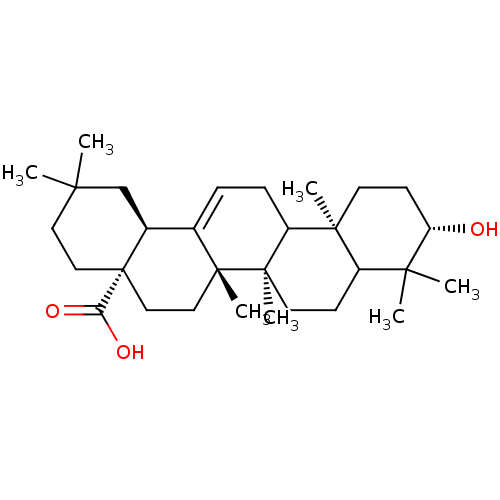 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242105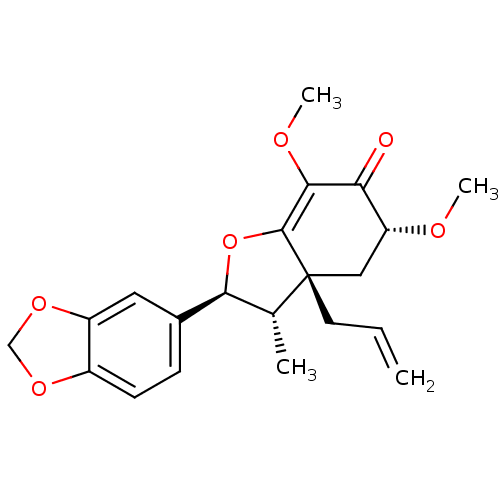 (CHEMBL470873 | armenin-B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50222205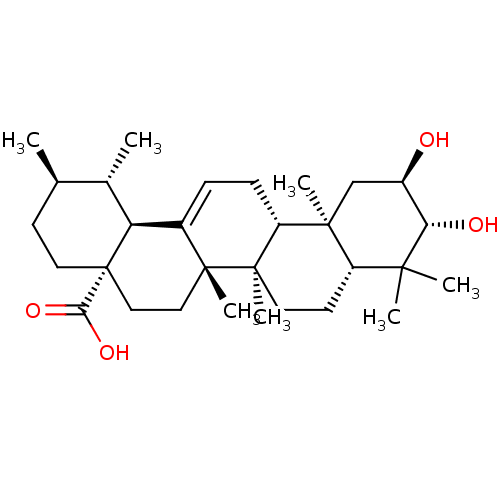 ((1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50250720 (3beta,16beta,23-triacetoxyolean-12-en-28-oic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242088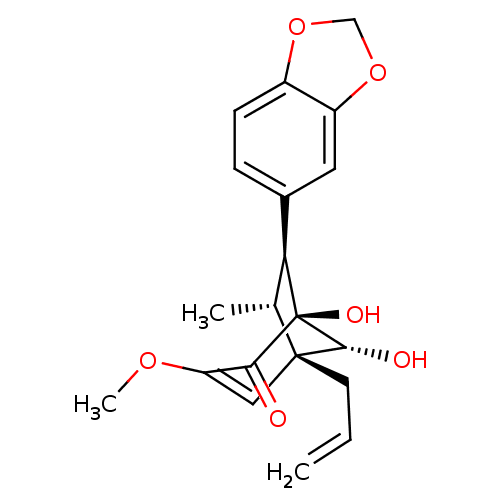 ((7S,8R,1'S,5'S,6'R)-delta 2',8'-5',6'-Dihydroxy-3'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM23417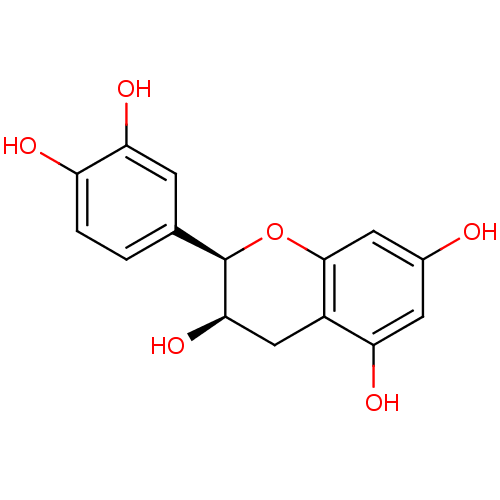 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of lyase activity of rat DNA polymerase beta after 30 mins by deoxyribose phosphate excision assay | J Nat Prod 67: 1744-7 (2004) Article DOI: 10.1021/np040057p BindingDB Entry DOI: 10.7270/Q2251K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242090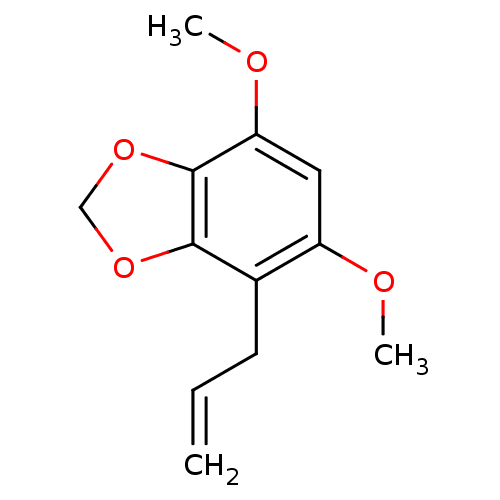 (2,4-Dimethoxy-5,6-methylenedioxy-1-(2-propenyl)ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242129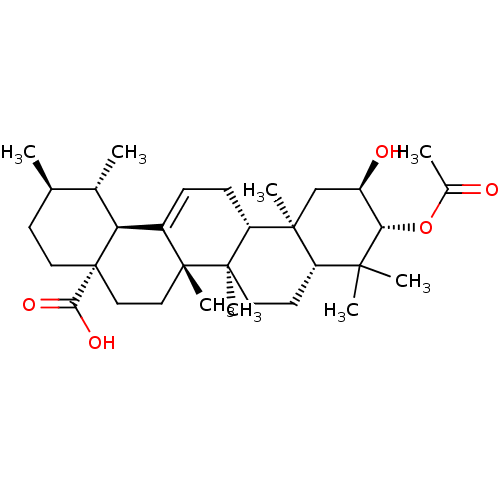 (3-beta-acetoxy-2-alpha-hydroxyurs-12-en-28-oic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242104 ((7S,8R,1'S,5'S,6'R)-delta 2',8'-3',6'-dihydroxy-5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50218197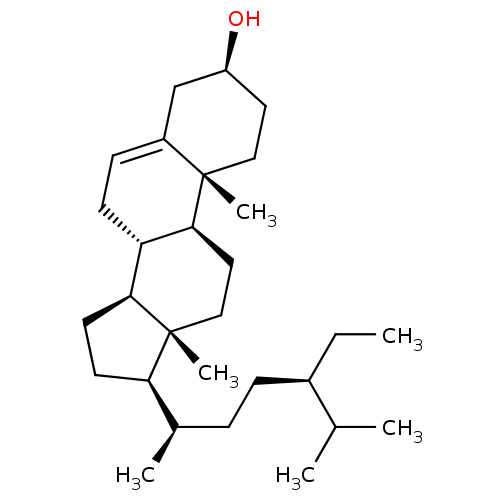 ((-)-beta-Sitosterol | (24R)-Ethylcholest-5-en-3bet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242092 (CHEMBL511441 | canellin A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50257635 ((-)-BETA-SITOSTEROL-BETA-D-GLUCOPYRANOSIDE | (2R,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242093 (CHEMBL469849 | canellin C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242107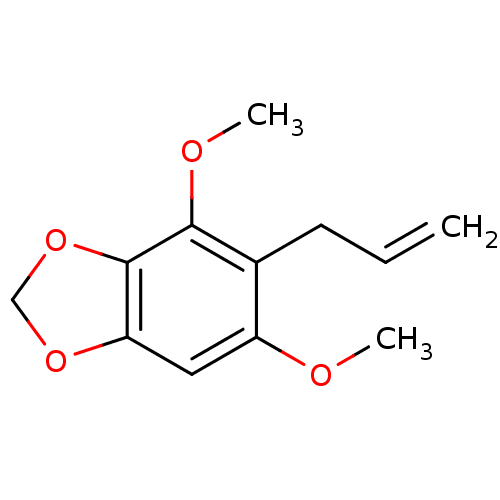 (1-allyl-2,6-dimethoxy-3,4-methylenedioxybenzene | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50376364 (STIGMASTEROL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242091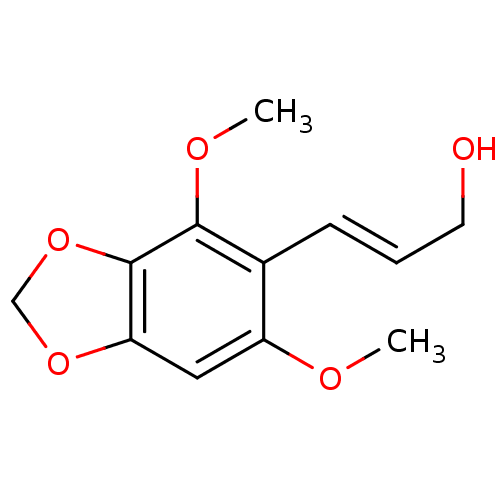 (2,6-Dimethoxy-3,4-methylenedioxycinnamyl alcohol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM23208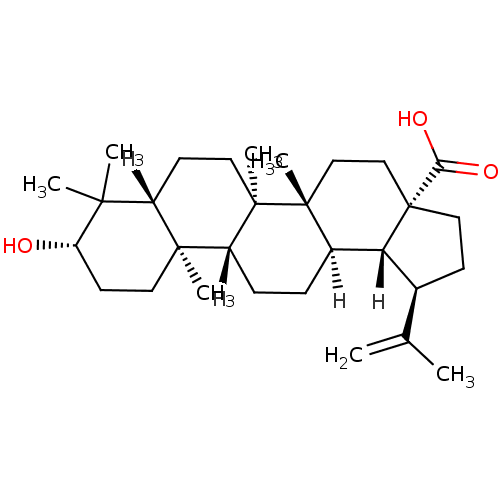 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay | J Nat Prod 66: 1463-5 (2003) Article DOI: 10.1021/np0301893 BindingDB Entry DOI: 10.7270/Q2K35VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242106 (CHEMBL470874 | dillapiol | dillapiole) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242103 (3'-methoxyguianin | CHEMBL513395) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242135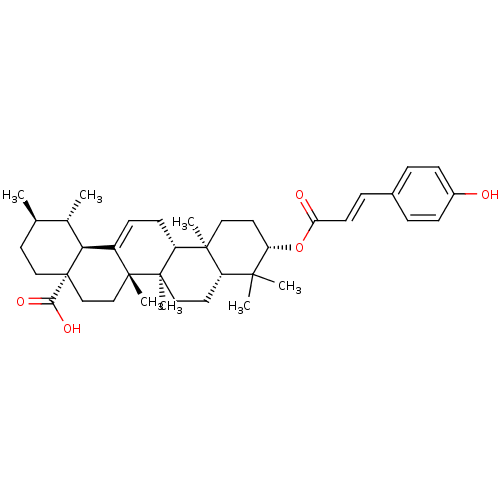 (3-(p-coumaroyl)ursolic acid | CHEMBL463917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 899-901 (2004) Article DOI: 10.1021/np030531b BindingDB Entry DOI: 10.7270/Q2KH0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242089 ((7S,8R,1'S,5'S,6'R)-delta 2',8'-3',5',6'-Trihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50242108 (CHEMBL506422 | omega-hydroxyisodillapiole) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of DNA polymerase beta (unknown origin) lyase activity | J Nat Prod 67: 964-7 (2004) Article DOI: 10.1021/np030507y BindingDB Entry DOI: 10.7270/Q2FQ9WDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50260079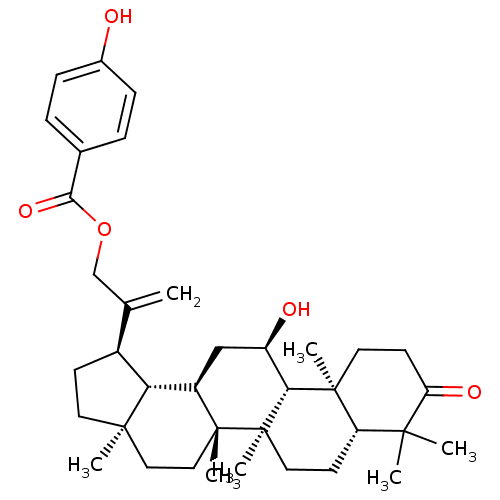 (30-(4'-hydroxybenzoyloxy)-11alpha-hydroxylupane-20...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of lyase activity of rat DNA polymerase beta after 30 mins by deoxyribose phosphate excision assay | J Nat Prod 67: 1744-7 (2004) Article DOI: 10.1021/np040057p BindingDB Entry DOI: 10.7270/Q2251K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||