

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276796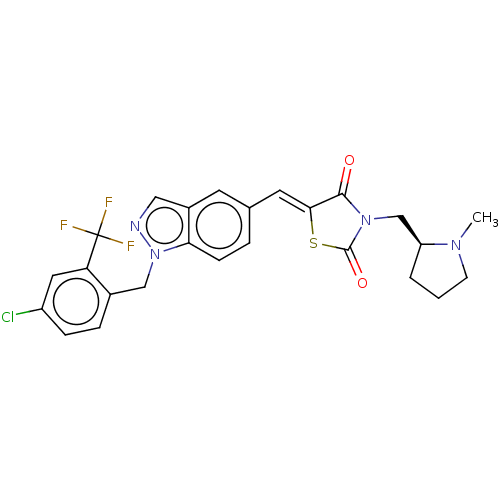 (CHEMBL4170306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50276802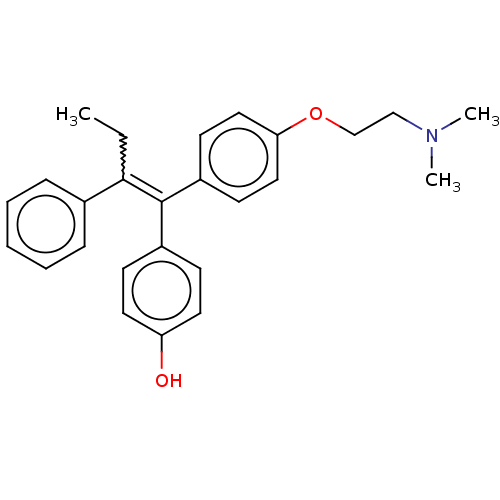 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 green binding to recombinant full length human ERalpha expressed in insect cells by fluorescence polarization assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to recombinant full length human ERbeta expressed in insect cells by fluorescence polarization assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276805 (CHEMBL4166492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276818 (CHEMBL4161346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276803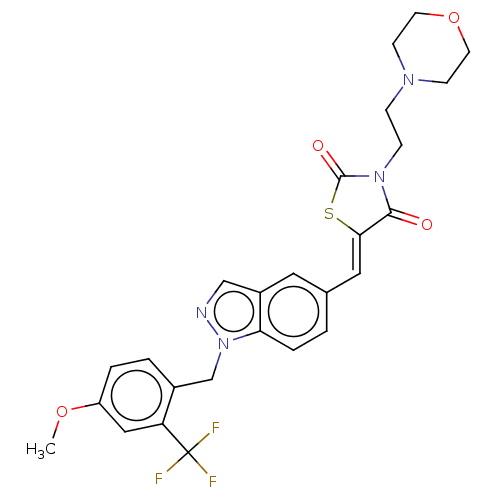 (CHEMBL4169272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276817 (CHEMBL4159613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276834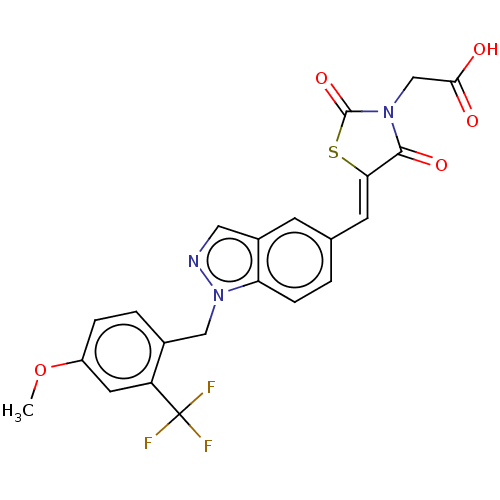 (CHEMBL4163909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50276802 (4-OHT | Afimoxifene | TamoGel) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at ERRgamma (unknown origin) assessed as inhibition of co-activator peptide recruitment by TR-FRET assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276816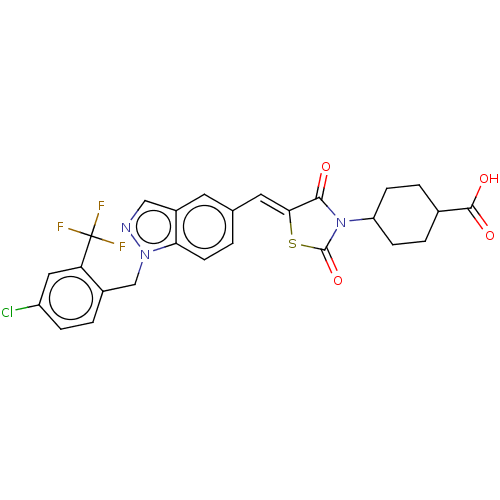 (CHEMBL4166832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276822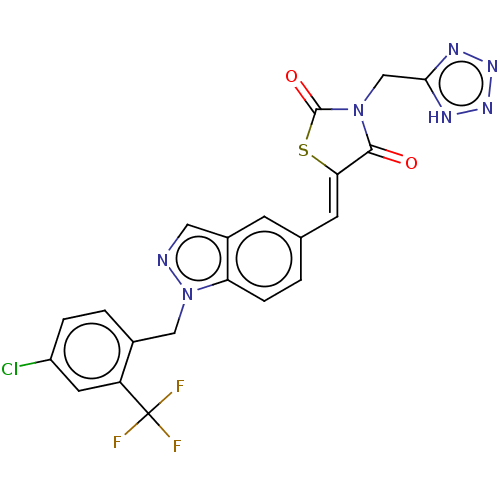 (CHEMBL4165363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336739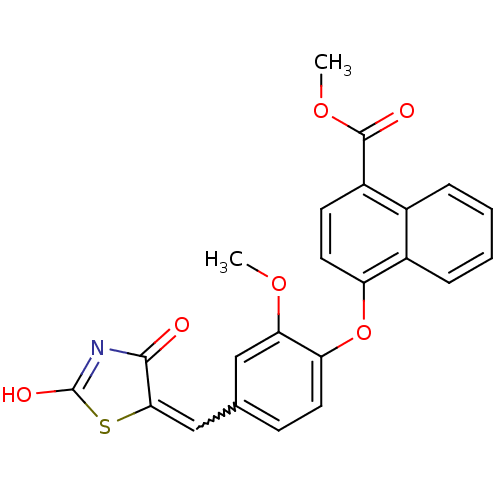 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276830 (CHEMBL4172209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276811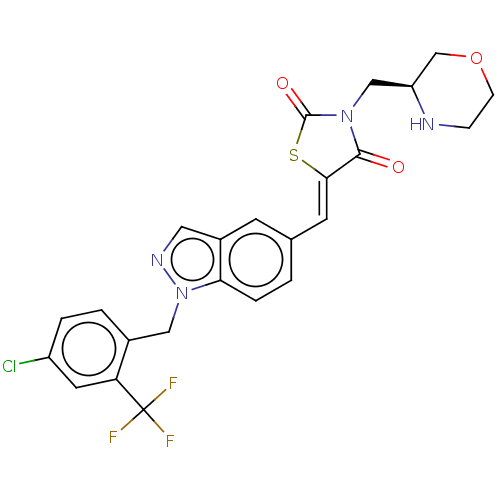 (CHEMBL4172522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336759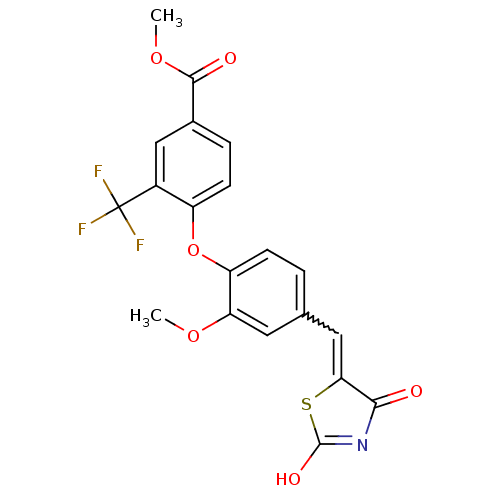 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276814 (CHEMBL4163054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276795 (CHEMBL4163375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276835 (CHEMBL4173278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276823 (CHEMBL4177113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276836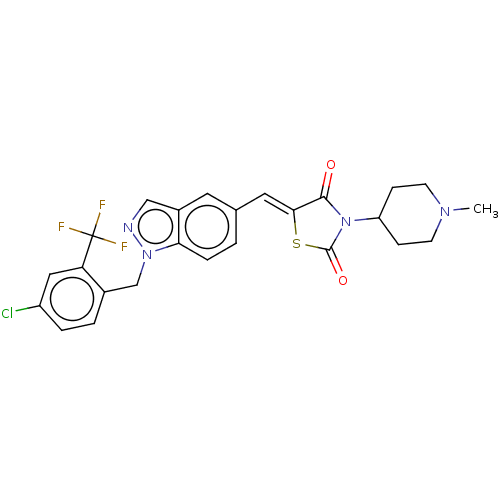 (CHEMBL4173757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276812 (CHEMBL4164196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276797 (CHEMBL4174856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276833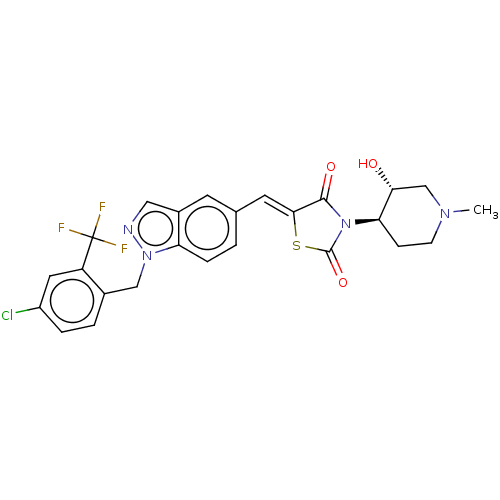 (CHEMBL4175927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276831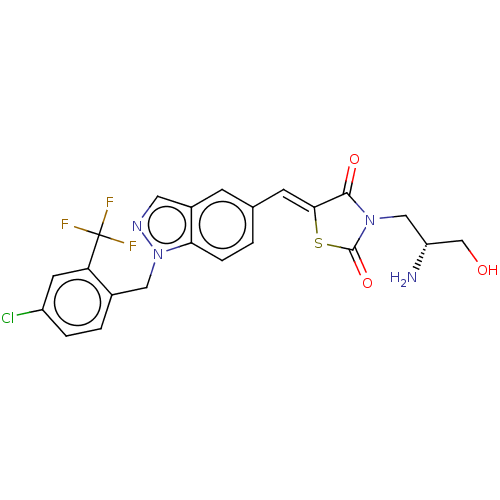 (CHEMBL4164640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276824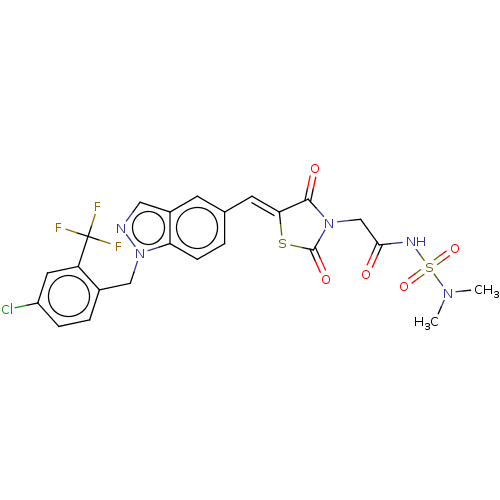 (CHEMBL4169919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276825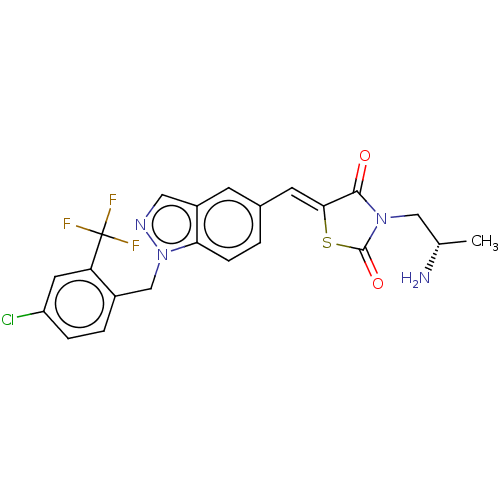 (CHEMBL4161594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276810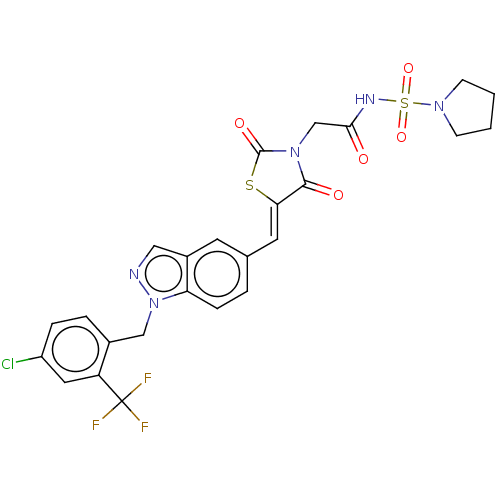 (CHEMBL4161992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276832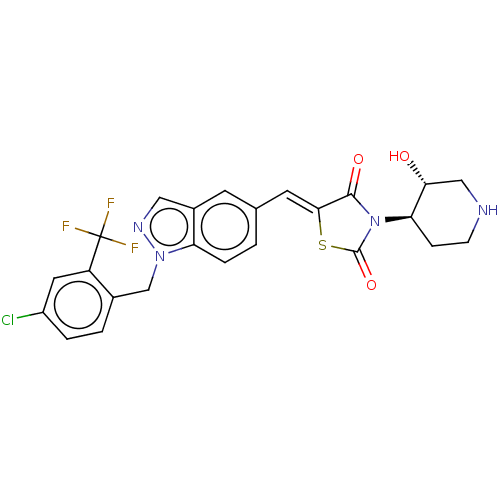 (CHEMBL4168072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276804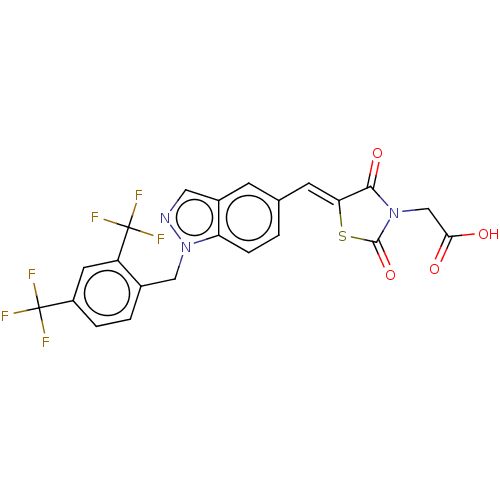 (CHEMBL4171282) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276821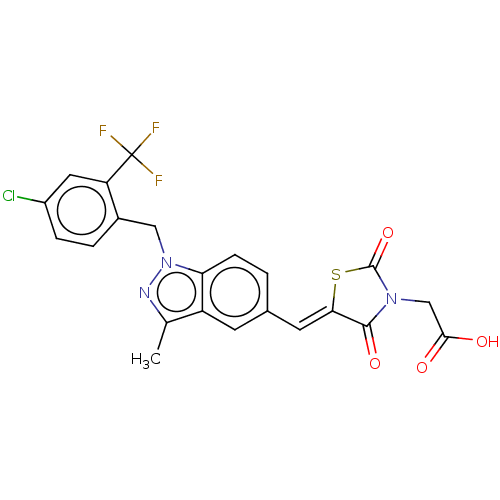 (CHEMBL4167938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276815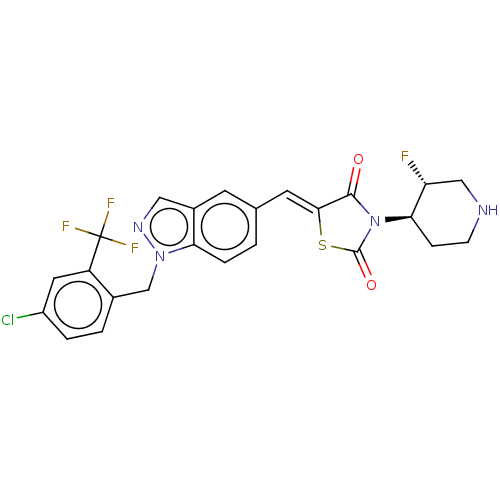 (CHEMBL4171419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336760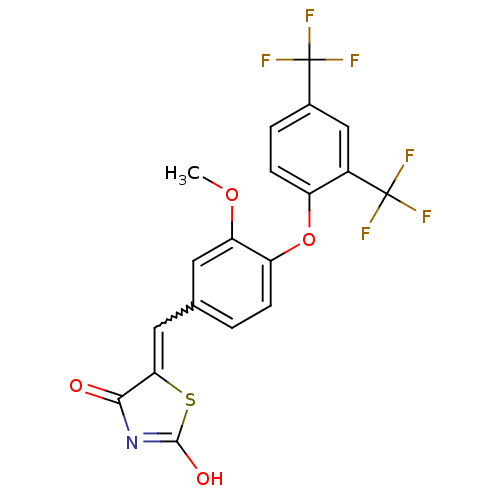 (5-[4-(2,4-Bis-trifluoromethylphenoxy)-3-methoxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276819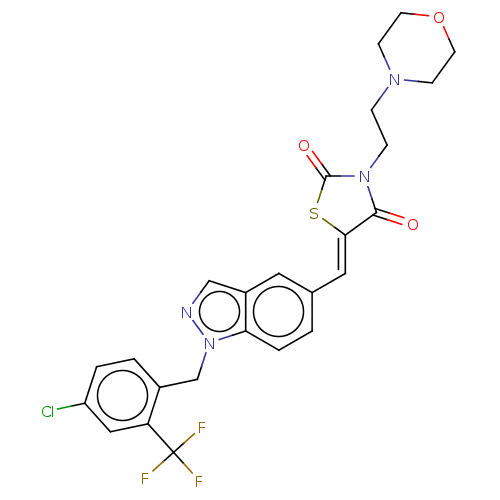 (CHEMBL4174736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276838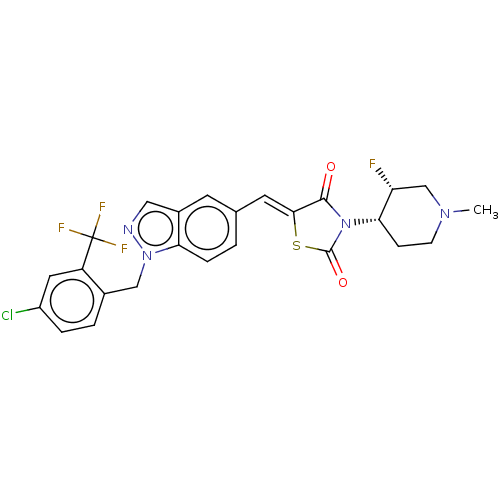 (CHEMBL4160799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276813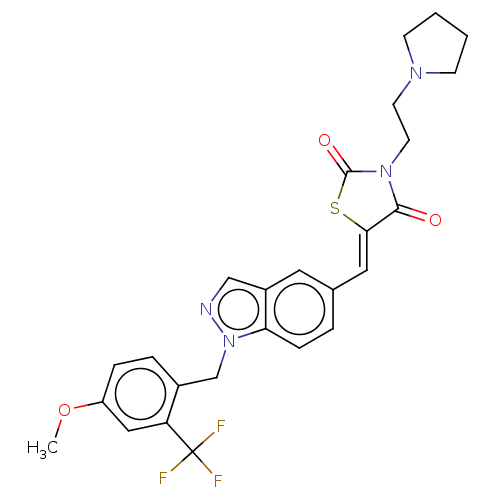 (CHEMBL4170236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336753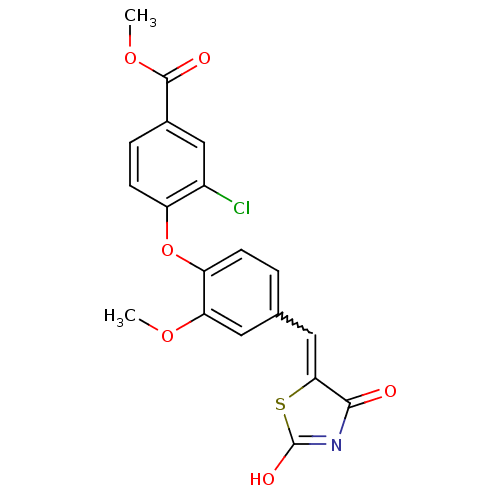 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276837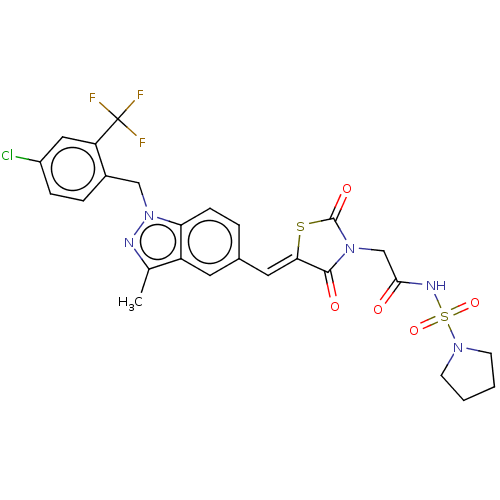 (CHEMBL4159675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336731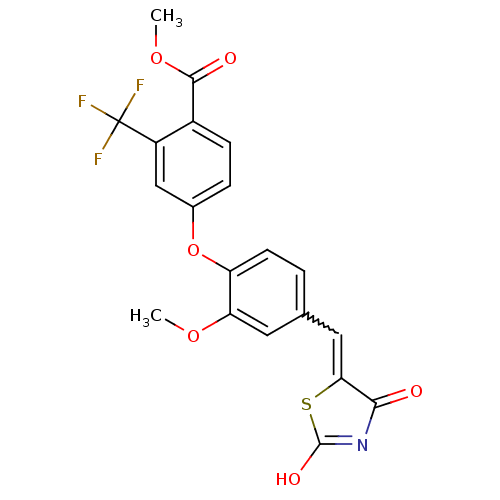 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336738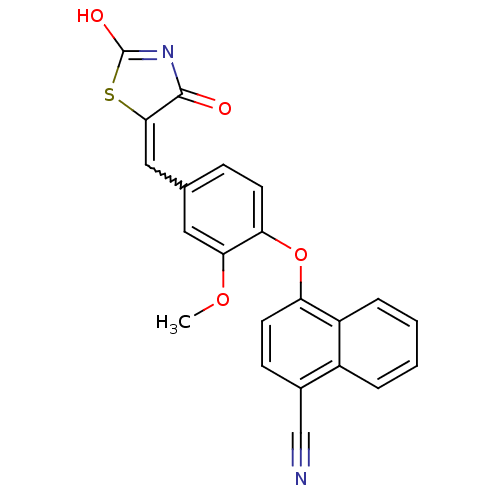 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336761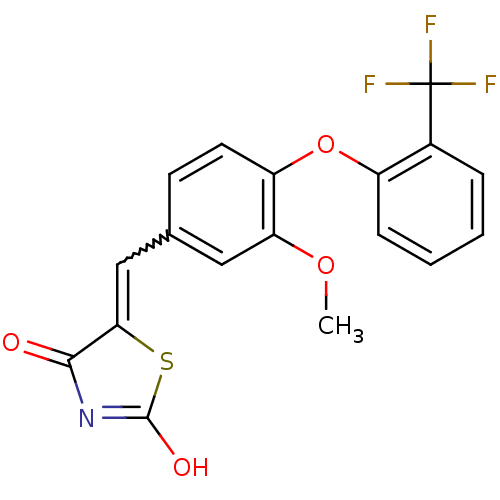 (5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336762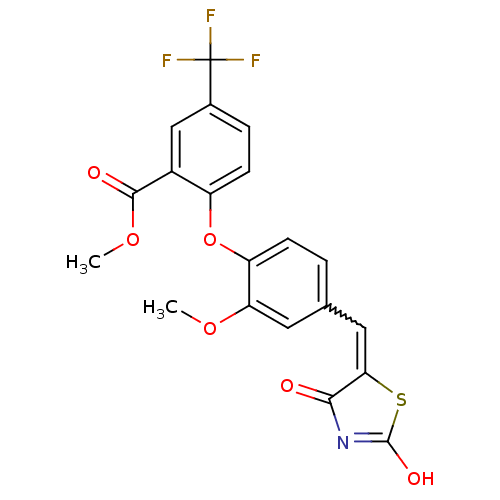 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336756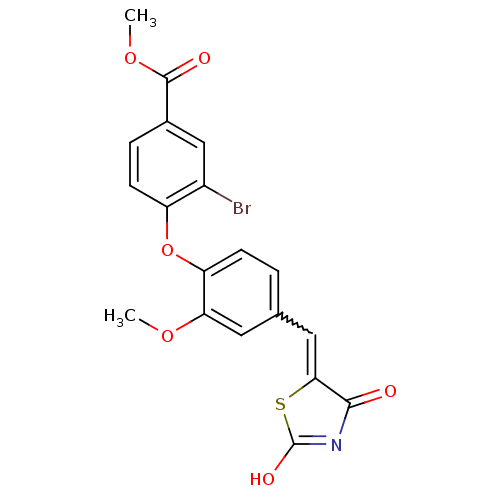 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336739 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474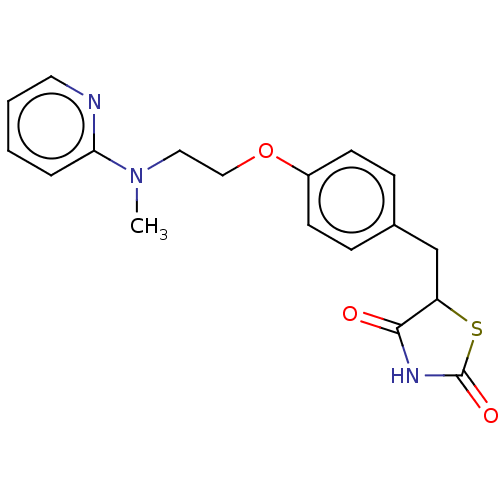 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone PPARgamma Green binding to recombinant N-terminal GST-tagged human PPARgamma LBD by fluorescence polarization assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336757 (5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336754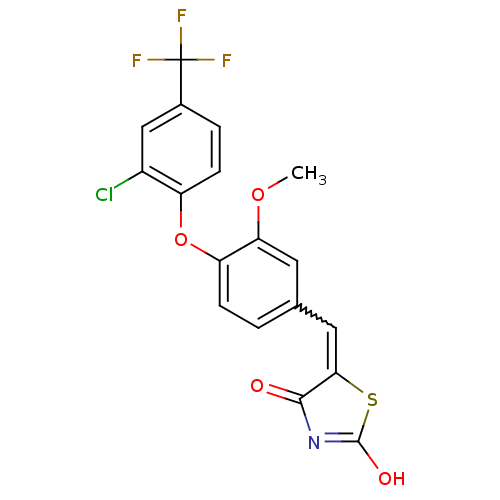 (5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM22420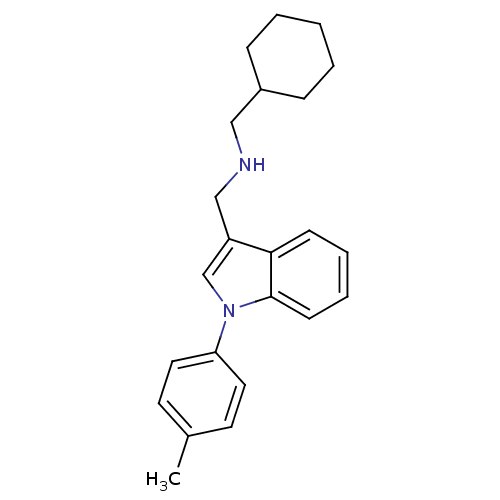 ((cyclohexylmethyl)({[1-(4-methylphenyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336735 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336752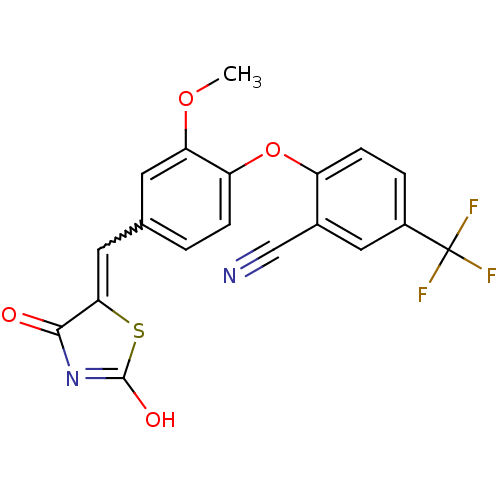 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 171 total ) | Next | Last >> |