

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50218464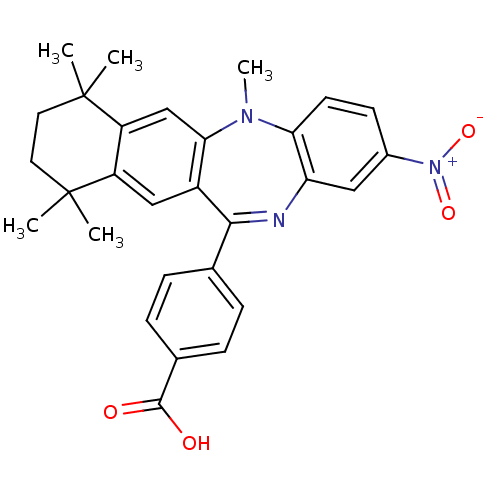 ((Z)-4-(5,7,7,10,10-pentamethyl-2-nitro-7,8,9,10-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50476882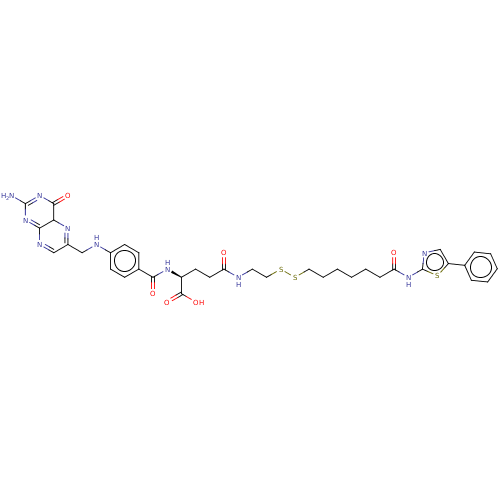 (CHEMBL428721) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells in presence of DTT after 30 mins by fluorescent activity assay | Bioorg Med Chem Lett 17: 4208-12 (2007) Article DOI: 10.1016/j.bmcl.2007.05.040 BindingDB Entry DOI: 10.7270/Q2GH9MRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50218464 ((Z)-4-(5,7,7,10,10-pentamethyl-2-nitro-7,8,9,10-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50218464 ((Z)-4-(5,7,7,10,10-pentamethyl-2-nitro-7,8,9,10-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256177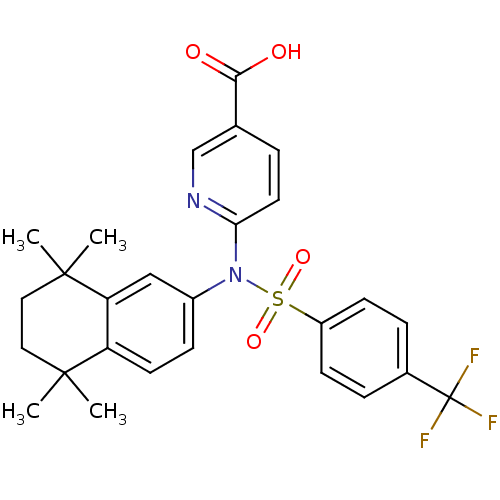 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256176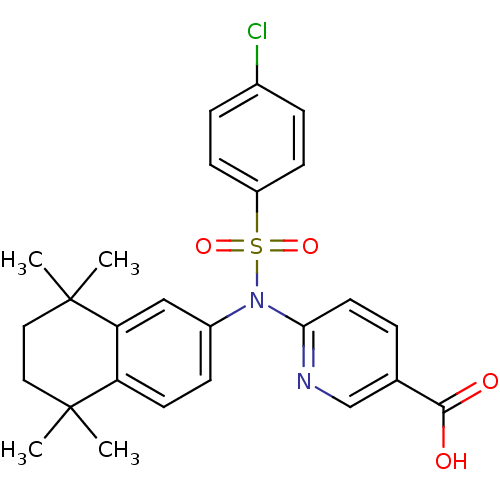 (6-(4-chloro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256175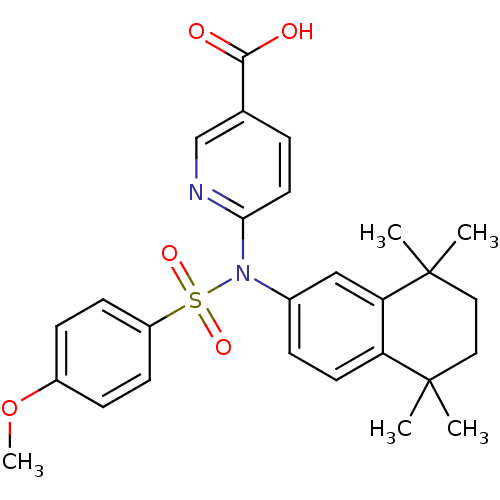 (6-(4-methoxy-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256177 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256174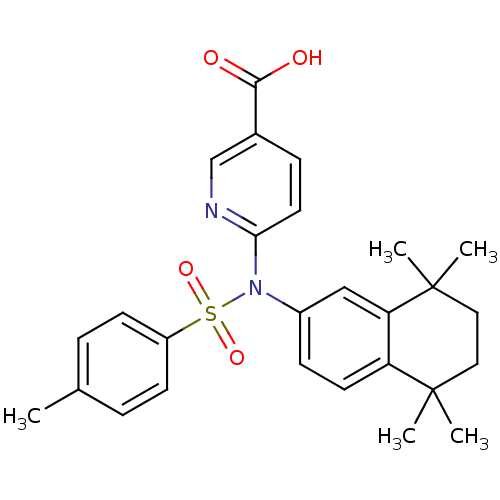 (6-(4-methyl-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50476883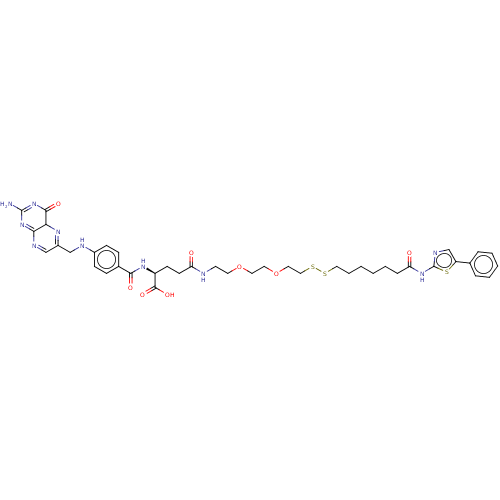 (CHEMBL235880) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells in presence of DTT after 30 mins by fluorescent activity assay | Bioorg Med Chem Lett 17: 4208-12 (2007) Article DOI: 10.1016/j.bmcl.2007.05.040 BindingDB Entry DOI: 10.7270/Q2GH9MRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256177 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256174 (6-(4-methyl-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256175 (6-(4-methoxy-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256176 (6-(4-chloro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256176 (6-(4-chloro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256174 (6-(4-methyl-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256175 (6-(4-methoxy-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50476884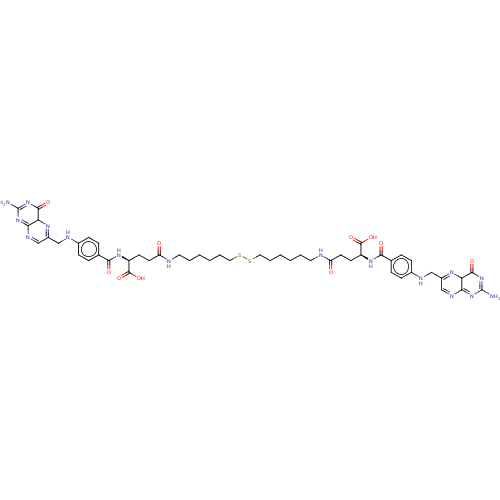 (CHEMBL236723) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells in presence of DTT after 30 mins by fluorescent activity assay | Bioorg Med Chem Lett 17: 4208-12 (2007) Article DOI: 10.1016/j.bmcl.2007.05.040 BindingDB Entry DOI: 10.7270/Q2GH9MRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256124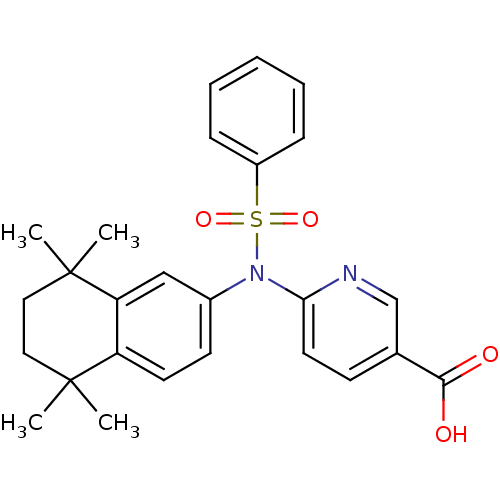 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256124 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256124 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256275 (6-(4-nitro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256123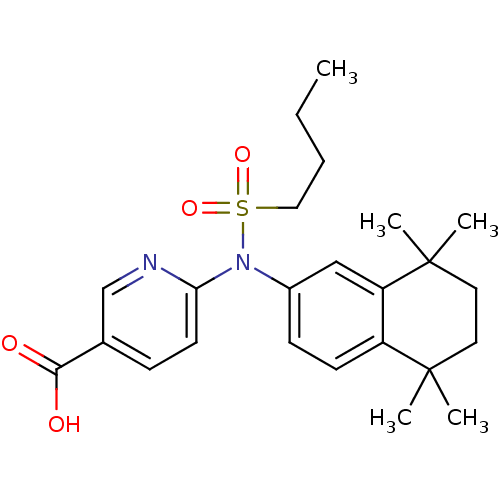 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50256275 (6-(4-nitro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRgamma (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256275 (6-(4-nitro-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50256123 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRbeta (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activi... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50256123 (6-(N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at RXRalpha (unknown origin) expressed in african green monkey COS1 cells assessed as inhibition of LGD1069-induced agonist activ... | Bioorg Med Chem Lett 19: 1001-3 (2009) Article DOI: 10.1016/j.bmcl.2008.11.086 BindingDB Entry DOI: 10.7270/Q2TH8MJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50476881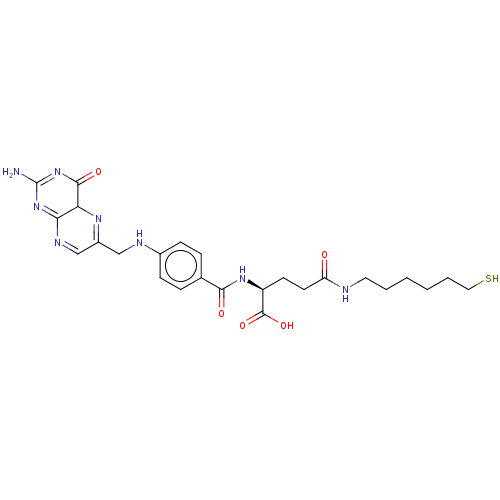 (CHEMBL438153) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells after 30 mins by fluorescent activity assay | Bioorg Med Chem Lett 17: 4208-12 (2007) Article DOI: 10.1016/j.bmcl.2007.05.040 BindingDB Entry DOI: 10.7270/Q2GH9MRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||