

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179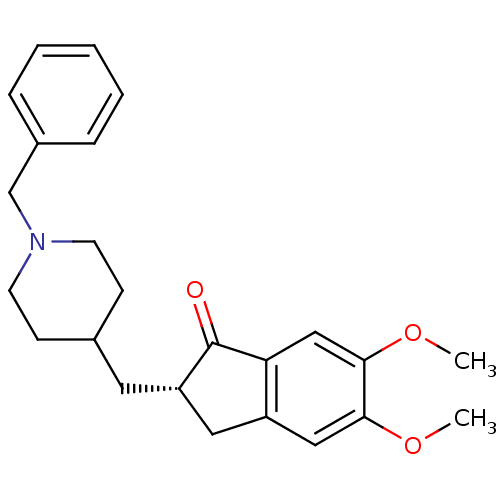 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941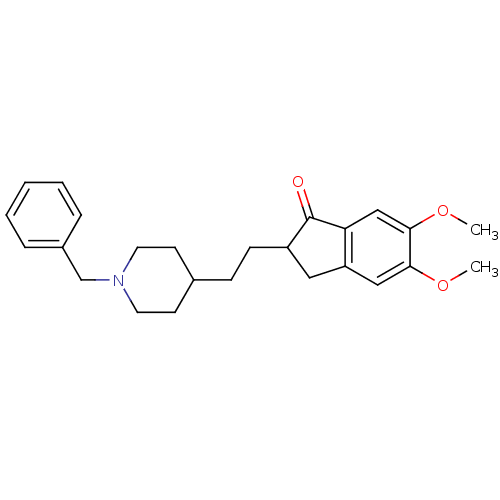 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422179 (CHEMBL2093912) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029941 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,6-dimethox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-2) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50029912 (2-(1-Benzyl-piperidin-4-yl)-5,6-dimethoxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity was calculated for the model Acetylcholinesterase (Expt-1) | J Med Chem 39: 4460-70 (1996) Article DOI: 10.1021/jm950596e BindingDB Entry DOI: 10.7270/Q2HD7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9410 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-ethyl-4-(phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9411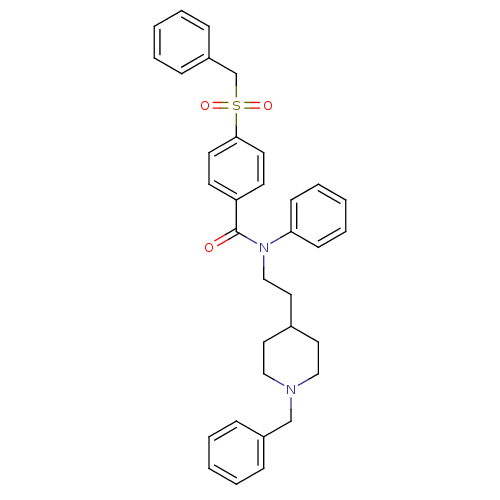 (CHEMBL54058 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409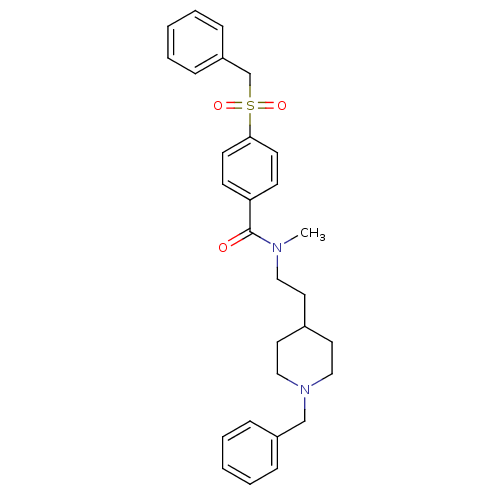 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9397 (CHEMBL59583 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408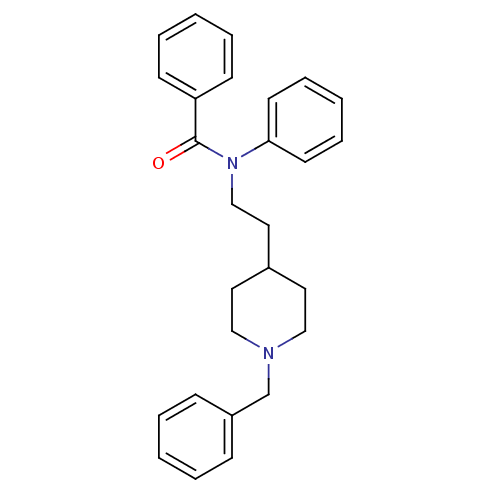 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9400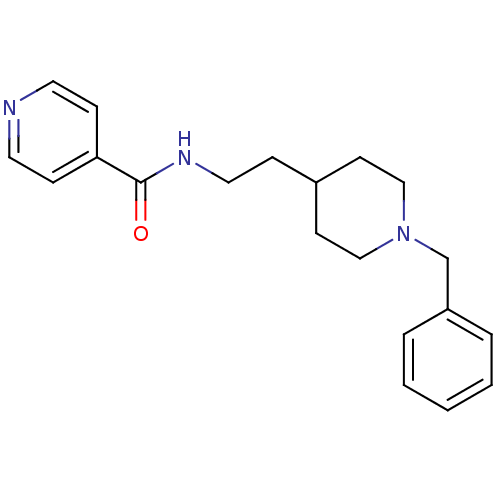 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9396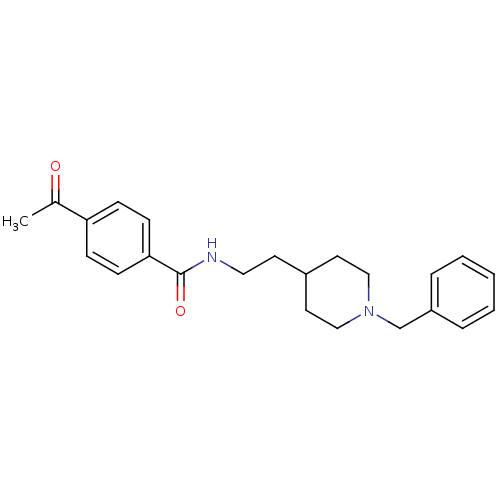 (CHEMBL56001 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9391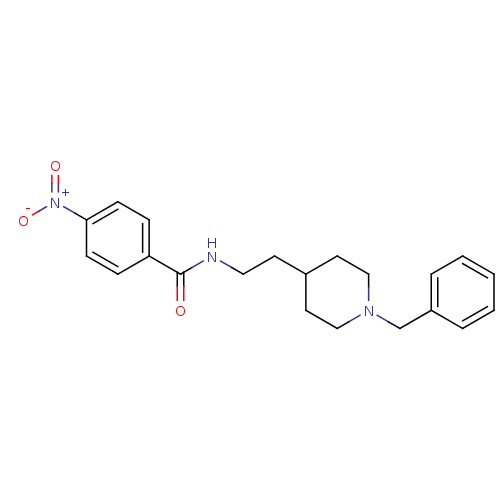 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-4-nitrobenzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9399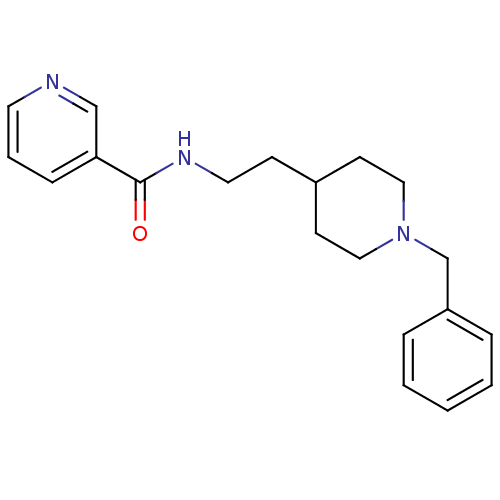 (CHEMBL299313 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9395 (CHEMBL56706 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9392 (CHEMBL54835 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9393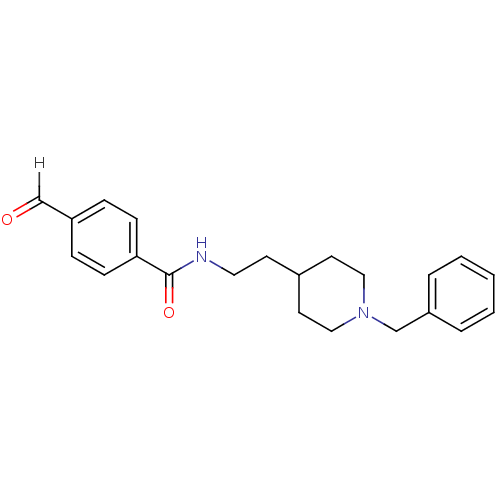 (CHEMBL56586 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9406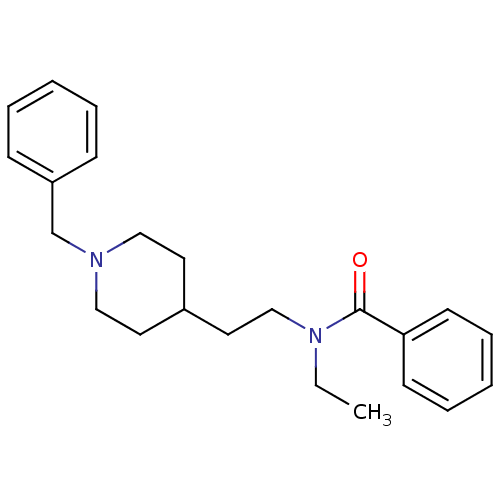 (CHEMBL57696 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9413 (CHEMBL127750 | N-methyl-N-(2-{1-[(3-methylphenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9401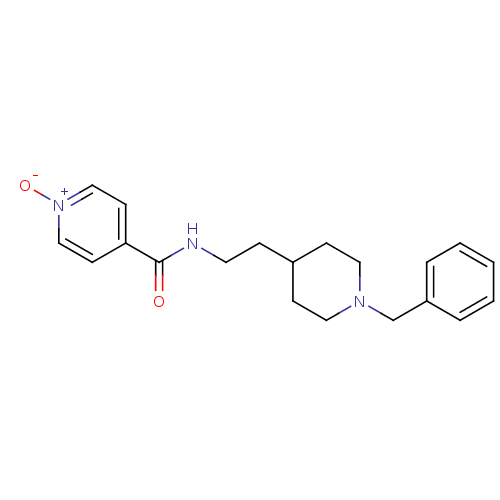 (4-{[2-(1-benzylpiperidin-4-yl)ethyl]carbamoyl}-1-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9405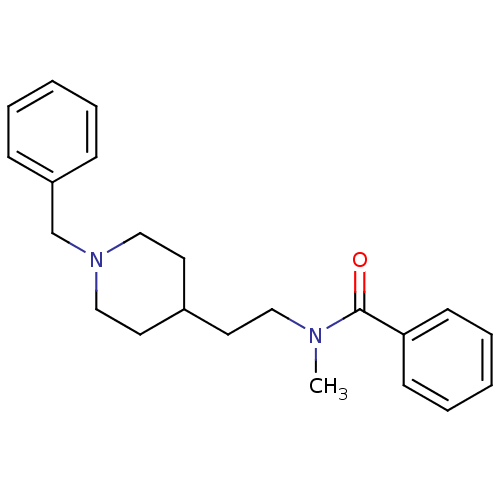 (CHEMBL56470 | N-[2-(1-Benzyl-piperidin-4-yl)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9394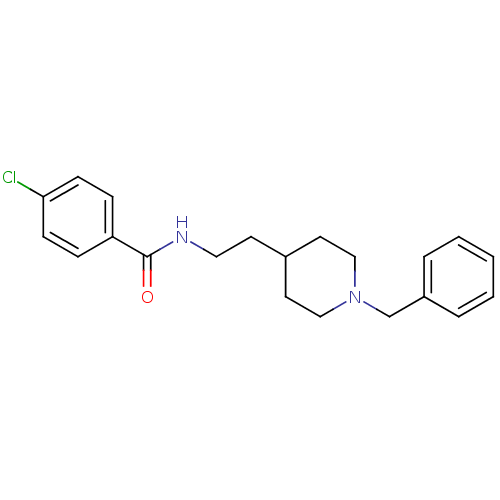 (CHEMBL55253 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9388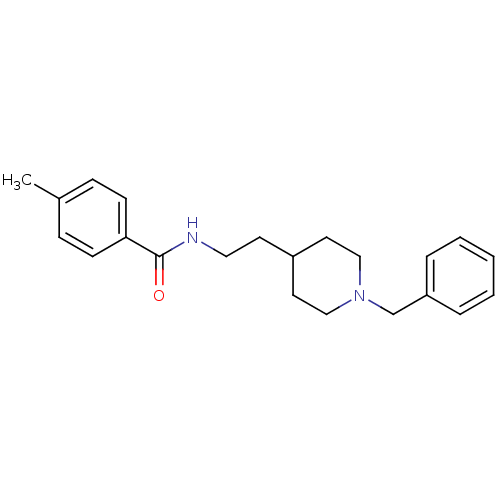 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-4-methylbenzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9390 (CHEMBL55466 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9416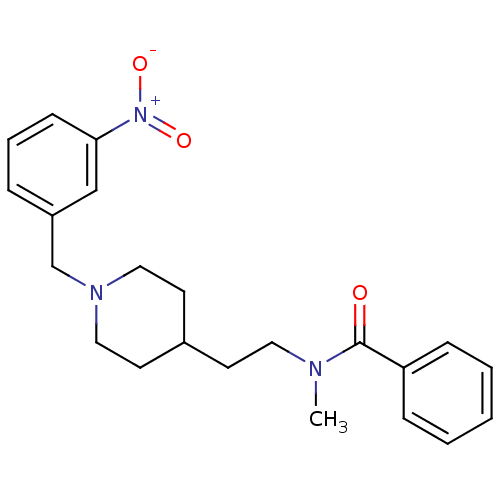 (CHEMBL126827 | N-methyl-N-(2-{1-[(3-nitrophenyl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9423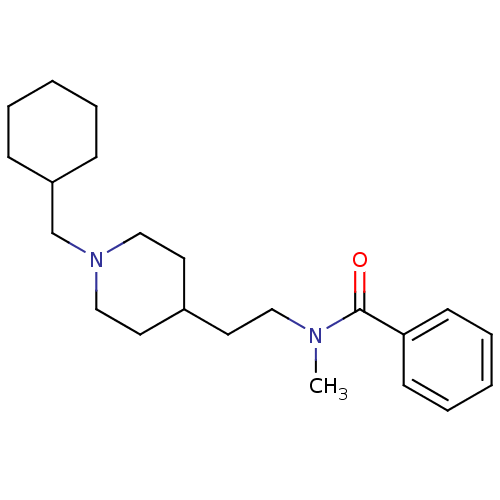 (CHEMBL419782 | N-{2-[1-(cyclohexylmethyl)piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9387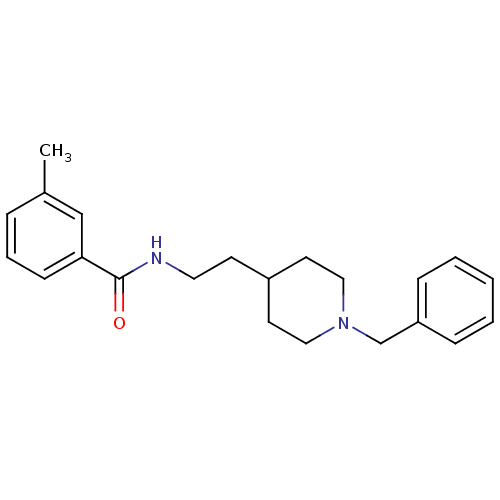 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-3-methylbenzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9403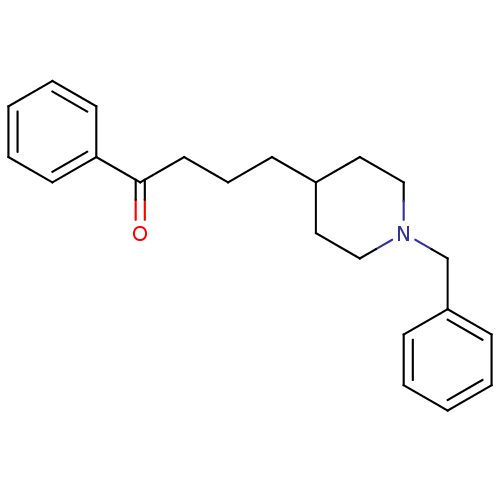 (1-Benzyl-4-(4-oxo-4-phenylbutyl)piperidine Hydroch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9385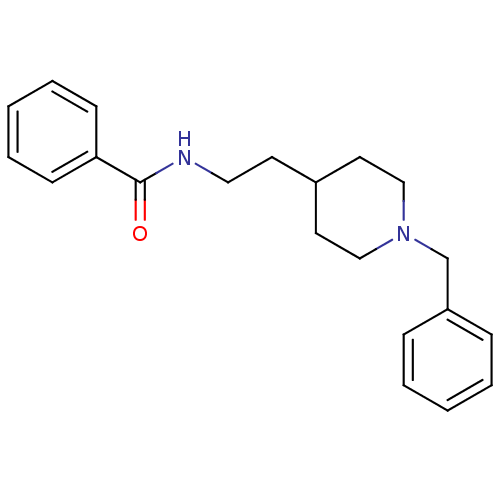 (1-Benzyl-4-[2-(N-benzoylamino)ethyl]piperidine Hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9412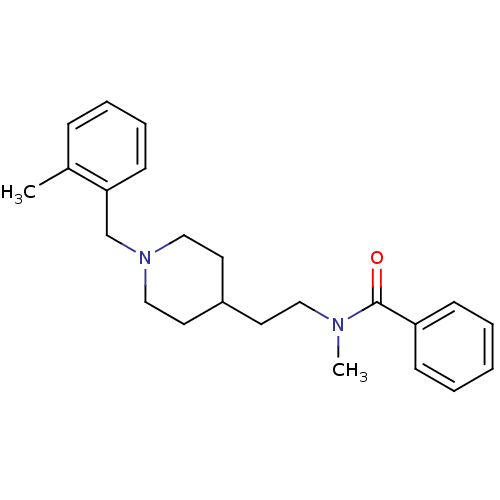 (CHEMBL126304 | N-methyl-N-(2-{1-[(2-methylphenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9398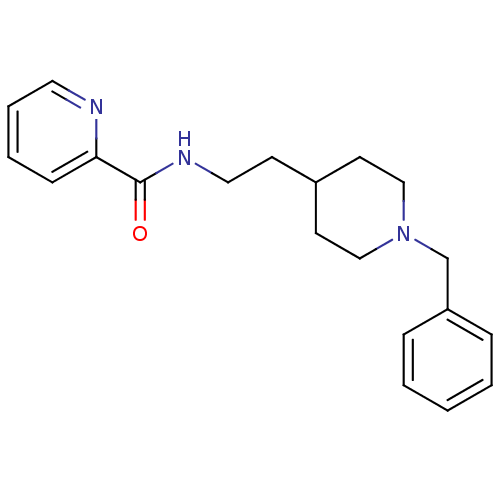 (CHEMBL56079 | N-[2-(1-benzylpiperidin-4-yl)ethyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9389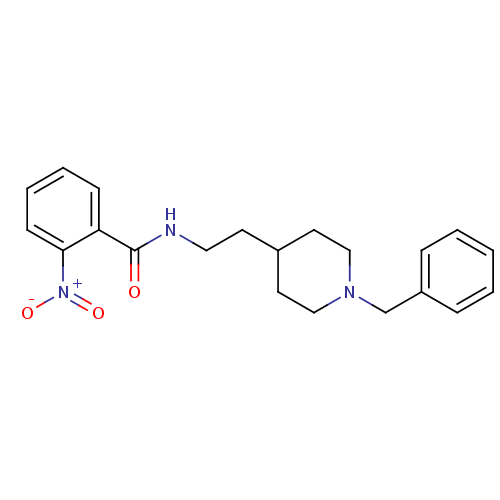 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9407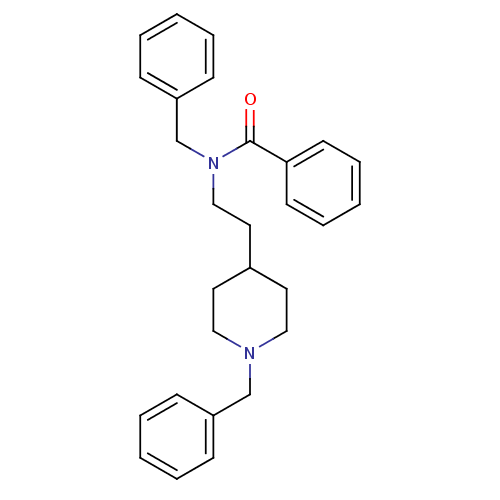 (1-Benzyl-4-[2-(N-benzoyl-N-benzylamino)ethyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9386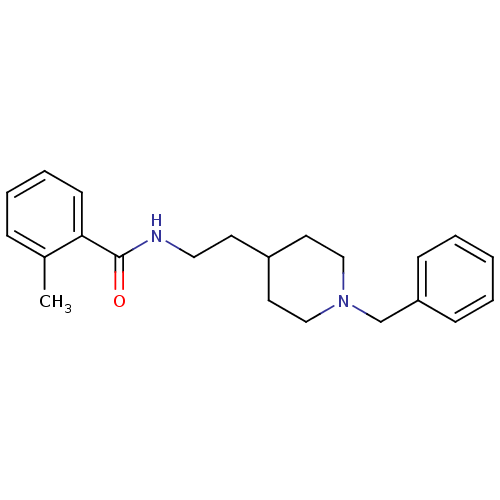 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-2-methylbenzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9402 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9417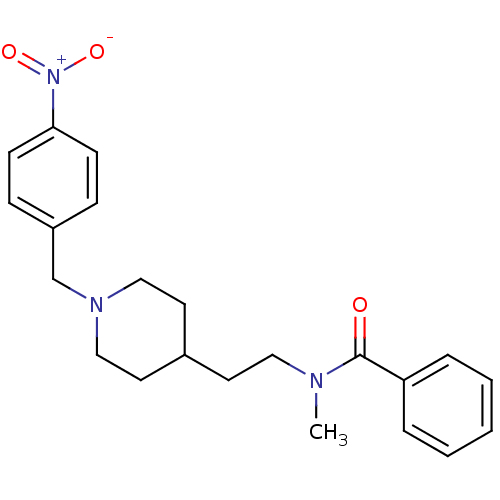 (CHEMBL125571 | N-methyl-N-(2-{1-[(4-nitrophenyl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9418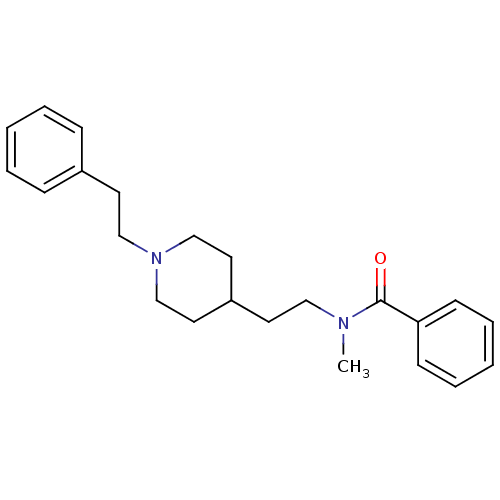 (N-methyl-N-{2-[1-(2-phenylethyl)piperidin-4-yl]eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9415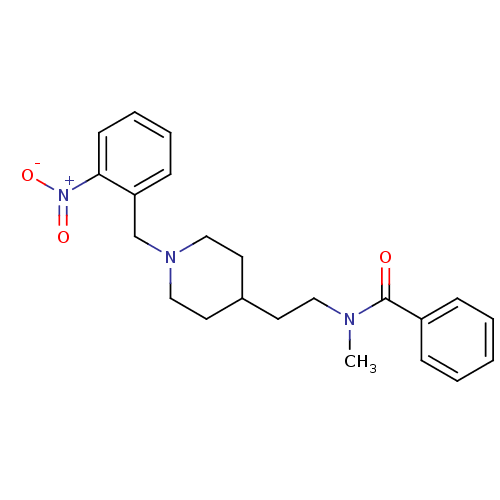 (CHEMBL340382 | N-methyl-N-(2-{1-[(2-nitrophenyl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9424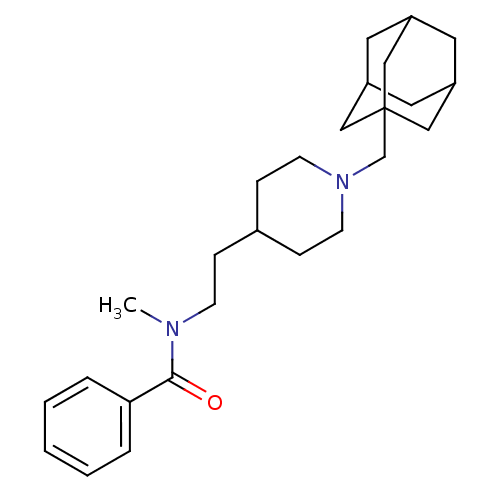 (N-{2-[1-(adamantan-1-ylmethyl)piperidin-4-yl]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9421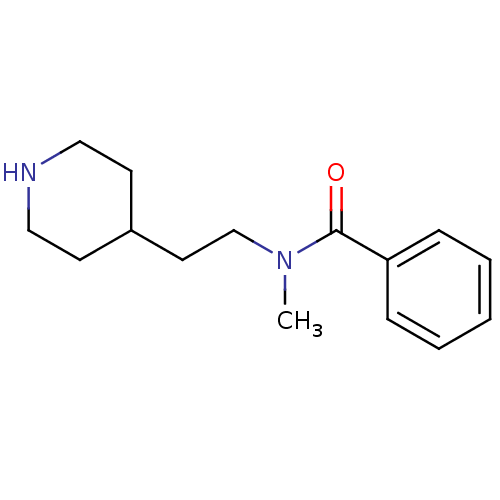 ((2E)-but-2-enedioic acid; N-methyl-N-[2-(piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9422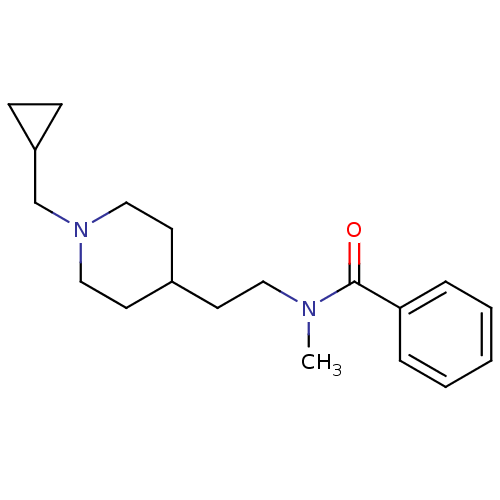 (CHEMBL126457 | N-{2-[1-(cyclopropylmethyl)piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9414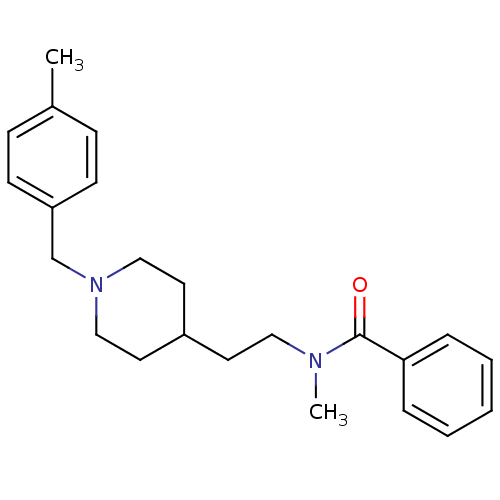 (CHEMBL127097 | N-methyl-N-(2-{1-[(4-methylphenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9404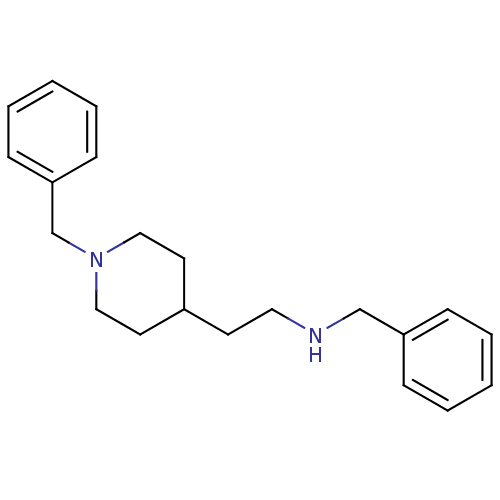 (Piperidine Derivative 12 | benzyl[2-(1-benzylpiper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |