 Found 15164 hits with Last Name = 'ke' and Initial = 'n'
Found 15164 hits with Last Name = 'ke' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M1 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50301066
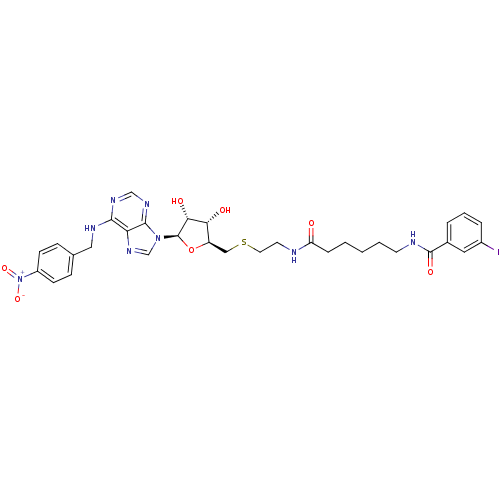
(CHEMBL571939 | N-(6-(2-(((2S,3S,4R,5R)-3,4-dihydro...)Show SMILES O[C@@H]1[C@@H](CSCCNC(=O)CCCCCNC(=O)c2cccc(I)c2)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)[N+]([O-])=O)ncnc12 |r| Show InChI InChI=1S/C32H37IN8O7S/c33-22-6-4-5-21(15-22)31(45)35-12-3-1-2-7-25(42)34-13-14-49-17-24-27(43)28(44)32(48-24)40-19-39-26-29(37-18-38-30(26)40)36-16-20-8-10-23(11-9-20)41(46)47/h4-6,8-11,15,18-19,24,27-28,32,43-44H,1-3,7,12-14,16-17H2,(H,34,42)(H,35,45)(H,36,37,38)/t24-,27-,28-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ulm University
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in HL60 cells by flow cytometry |
Bioorg Med Chem Lett 19: 5151-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.017
BindingDB Entry DOI: 10.7270/Q2QJ7HCF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M5 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880
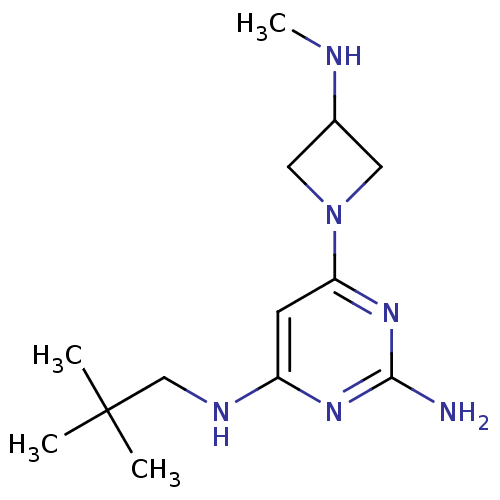
(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50245852
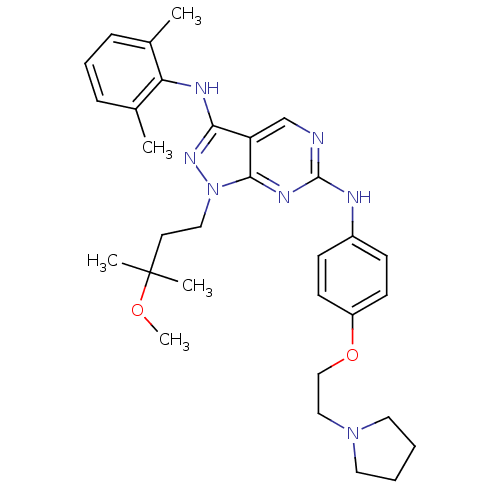
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50141614

((1S,2S,5S)-3-Hydroxy-4-[2-((S)-hydroxy-6-(S)-4-{(S...)Show SMILES CCC(C)(N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O Show InChI InChI=1S/C34H64N6O9/c1-12-34(11,35)33(49)40-29(20(8)9)32(48)39-28(19(6)7)31(47)38-22(13-17(2)3)24(41)15-26(43)36-21(10)30(46)37-23(14-18(4)5)25(42)16-27(44)45/h17-25,28-29,41-42H,12-16,35H2,1-11H3,(H,36,43)(H,37,46)(H,38,47)(H,39,48)(H,40,49)(H,44,45)/t21-,22-,23-,24-,25-,28-,29-,34?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against aspartyl protease pepsin |
Bioorg Med Chem Lett 14: 855-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.030
BindingDB Entry DOI: 10.7270/Q26H4GV5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355612
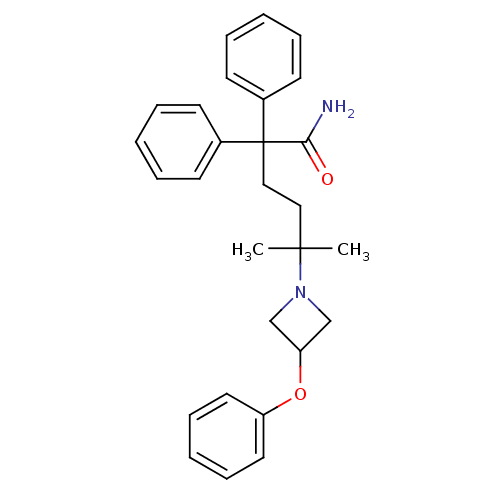
(CHEMBL1910848)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1ccccc1 Show InChI InChI=1S/C28H32N2O2/c1-27(2,30-20-25(21-30)32-24-16-10-5-11-17-24)18-19-28(26(29)31,22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-17,25H,18-21H2,1-2H3,(H2,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50355612

(CHEMBL1910848)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1ccccc1 Show InChI InChI=1S/C28H32N2O2/c1-27(2,30-20-25(21-30)32-24-16-10-5-11-17-24)18-19-28(26(29)31,22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-17,25H,18-21H2,1-2H3,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50301067
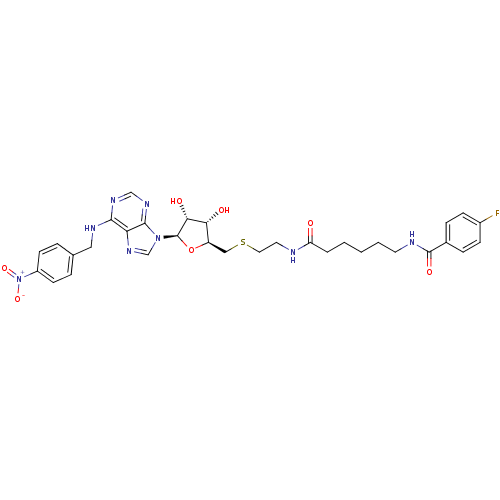
(CHEMBL569223 | N-(6-(2-(((2S,3S,4R,5R)-3,4-dihydro...)Show SMILES O[C@@H]1[C@@H](CSCCNC(=O)CCCCCNC(=O)c2ccc(F)cc2)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)[N+]([O-])=O)ncnc12 |r| Show InChI InChI=1S/C32H37FN8O7S/c33-22-9-7-21(8-10-22)31(45)35-13-3-1-2-4-25(42)34-14-15-49-17-24-27(43)28(44)32(48-24)40-19-39-26-29(37-18-38-30(26)40)36-16-20-5-11-23(12-6-20)41(46)47/h5-12,18-19,24,27-28,32,43-44H,1-4,13-17H2,(H,34,42)(H,35,45)(H,36,37,38)/t24-,27-,28-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ulm University
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in HL60 cells by flow cytometry |
Bioorg Med Chem Lett 19: 5151-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.017
BindingDB Entry DOI: 10.7270/Q2QJ7HCF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343152
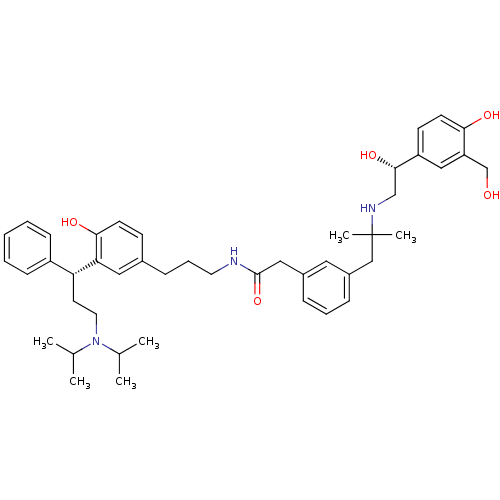
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17759
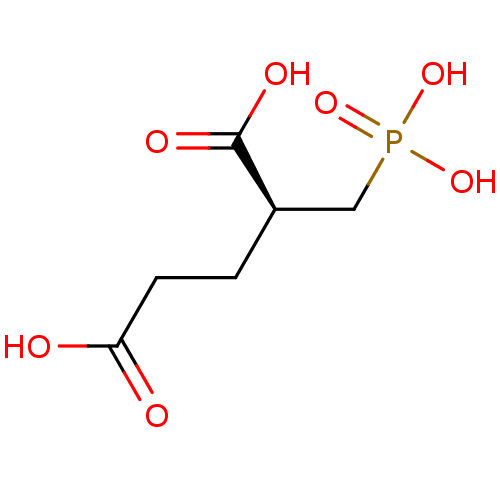
((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13)/t4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398821
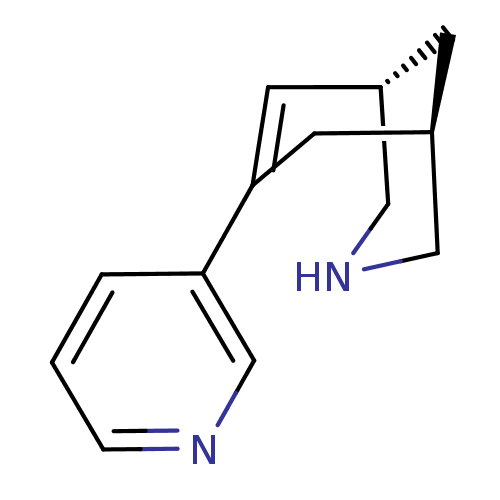
(CHEMBL2177537)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50397944
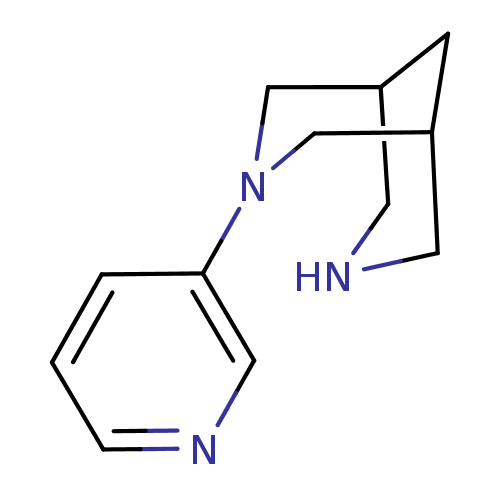
(CHEMBL2177512)Show InChI InChI=1S/C12H17N3/c1-2-12(7-13-3-1)15-8-10-4-11(9-15)6-14-5-10/h1-3,7,10-11,14H,4-6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355612

(CHEMBL1910848)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1ccccc1 Show InChI InChI=1S/C28H32N2O2/c1-27(2,30-20-25(21-30)32-24-16-10-5-11-17-24)18-19-28(26(29)31,22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-17,25H,18-21H2,1-2H3,(H2,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M1 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050807
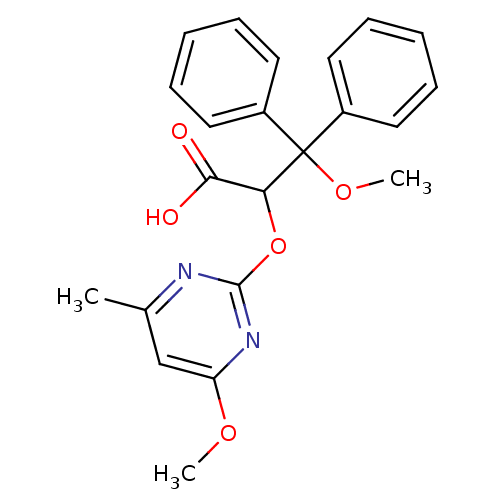
(3-Methoxy-2-(4-methoxy-6-methyl-pyrimidin-2-yloxy)...)Show SMILES COc1cc(C)nc(OC(C(O)=O)C(OC)(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C22H22N2O5/c1-15-14-18(27-2)24-21(23-15)29-19(20(25)26)22(28-3,16-10-6-4-7-11-16)17-12-8-5-9-13-17/h4-14,19H,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Endothelin A receptor by measuring its ability to displace [125I]-ET-1 from CHO cells |
J Med Chem 39: 2123-8 (1996)
Article DOI: 10.1021/jm960274q
BindingDB Entry DOI: 10.7270/Q2G44PDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM92596
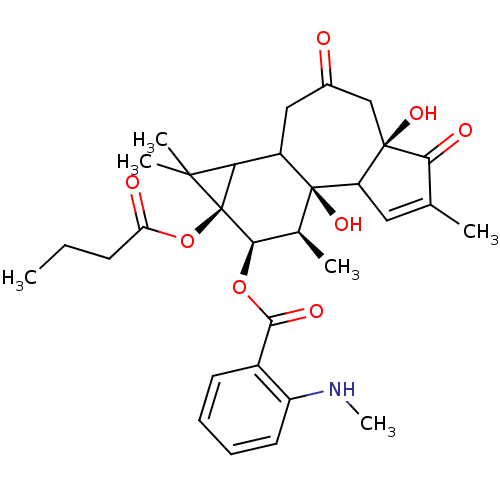
(Sapintoxin D)Show SMILES CCCC(=O)O[C@@]12C(C3CC(=O)C[C@@]4(O)C(C=C(C)C4=O)[C@]3(O)[C@@H](C)[C@H]1OC(=O)c1ccccc1NC)C2(C)C |r,t:16| Show InChI InChI=1S/C31H39NO8/c1-7-10-23(34)40-31-24(28(31,4)5)20-14-18(33)15-29(37)22(13-16(2)25(29)35)30(20,38)17(3)26(31)39-27(36)19-11-8-9-12-21(19)32-6/h8-9,11-13,17,20,22,24,26,32,37-38H,7,10,14-15H2,1-6H3/t17-,20?,22?,24?,26+,29+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health
| Assay Description
[3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. |
J Biol Chem 283: 10543-9 (2008)
Article DOI: 10.1074/jbc.M707463200
BindingDB Entry DOI: 10.7270/Q2CF9NQ6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50128835
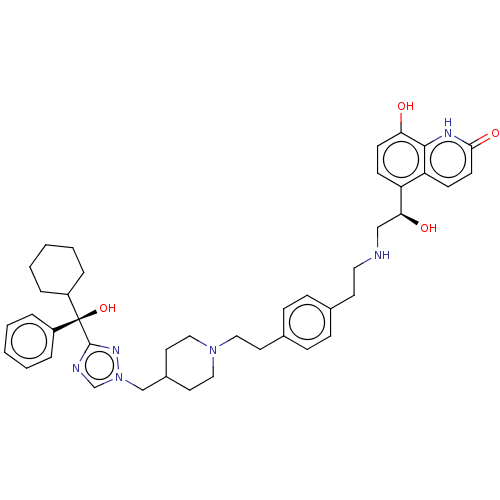
(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M2 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM85731
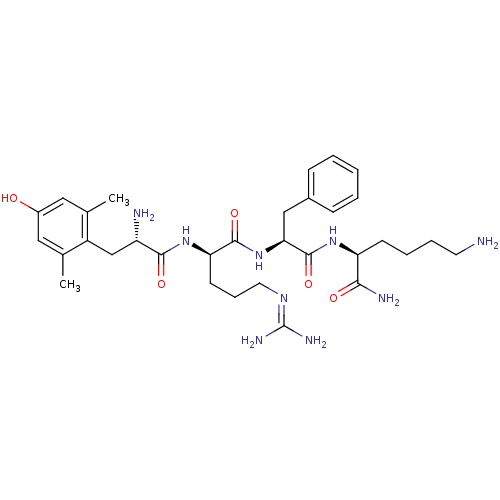
([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
J Med Chem 55: 9549-61 (2012)
Article DOI: 10.1021/jm3008079
BindingDB Entry DOI: 10.7270/Q2TM7C82 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM85731

([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs |
J Med Chem 55: 9549-61 (2012)
Article DOI: 10.1021/jm3008079
BindingDB Entry DOI: 10.7270/Q2TM7C82 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50346330
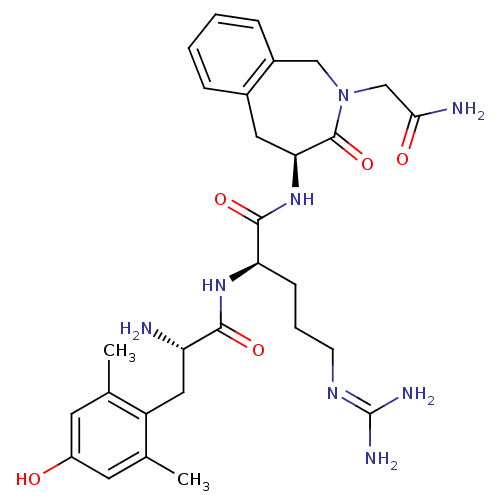
((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H]-1-[#6]-c2ccccc2-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6]-1=O |r| Show InChI InChI=1S/C29H40N8O5/c1-16-10-20(38)11-17(2)21(16)13-22(30)26(40)35-23(8-5-9-34-29(32)33)27(41)36-24-12-18-6-3-4-7-19(18)14-37(28(24)42)15-25(31)39/h3-4,6-7,10-11,22-24,38H,5,8-9,12-15,30H2,1-2H3,(H2,31,39)(H,35,40)(H,36,41)(H4,32,33,34)/t22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs |
J Med Chem 55: 9549-61 (2012)
Article DOI: 10.1021/jm3008079
BindingDB Entry DOI: 10.7270/Q2TM7C82 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355618
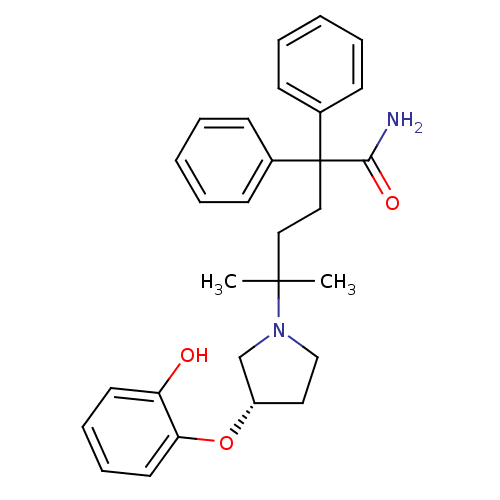
(CHEMBL1910852)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC[C@@H](C1)Oc1ccccc1O |r| Show InChI InChI=1S/C29H34N2O3/c1-28(2,31-20-17-24(21-31)34-26-16-10-9-15-25(26)32)18-19-29(27(30)33,22-11-5-3-6-12-22)23-13-7-4-8-14-23/h3-16,24,32H,17-21H2,1-2H3,(H2,30,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355631
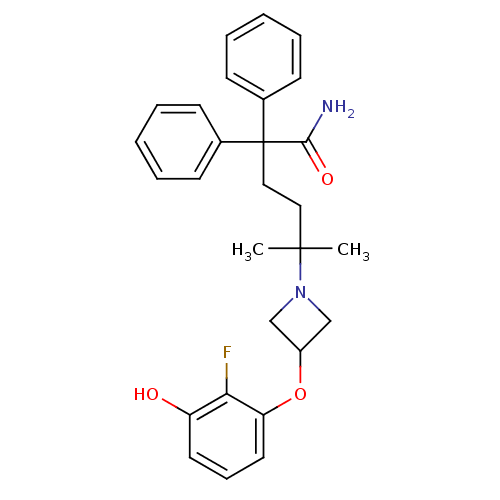
(CHEMBL1910865)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1F Show InChI InChI=1S/C28H31FN2O3/c1-27(2,31-18-22(19-31)34-24-15-9-14-23(32)25(24)29)16-17-28(26(30)33,20-10-5-3-6-11-20)21-12-7-4-8-13-21/h3-15,22,32H,16-19H2,1-2H3,(H2,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M5 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50194658

((2R)-N-(1-cyclopropylmethyl-4-piperidinylmethyl)-1...)Show SMILES O[C@@H]1C[C@H](N(C1)C(=O)CC(c1ccc(F)cc1)(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1CCC[C@@H]1C(=O)NCC1CCN(CC2CC2)CC1 Show InChI InChI=1S/C41H47F3N4O4/c42-32-11-5-29(6-12-32)41(30-7-13-33(43)14-8-30,31-9-15-34(44)16-10-31)23-38(50)48-26-35(49)22-37(48)40(52)47-19-1-2-36(47)39(51)45-24-27-17-20-46(21-18-27)25-28-3-4-28/h5-16,27-28,35-37,49H,1-4,17-26H2,(H,45,51)/t35-,36-,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells |
J Med Chem 49: 5653-63 (2006)
Article DOI: 10.1021/jm051205r
BindingDB Entry DOI: 10.7270/Q2F76C6K |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50358559
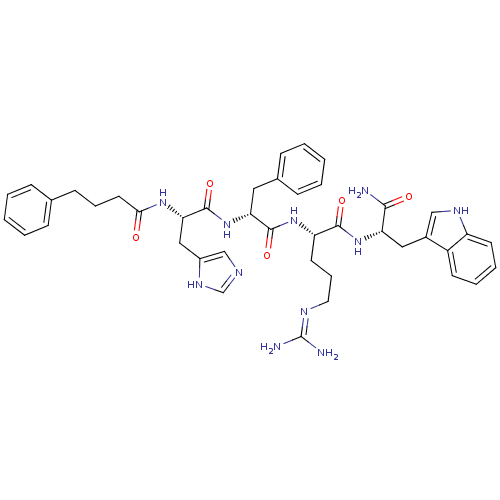
(CHEMBL1923662)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CCCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:11.19,7.7,22.30,wD:44.46,(13.28,5.22,;14.61,4.46,;15.94,5.22,;14.61,2.91,;13.27,2.14,;13.27,.61,;11.94,-.17,;11.94,-1.7,;10.61,-2.47,;9.27,-1.7,;9.27,-.16,;7.94,-2.47,;7.94,-4.01,;9.26,-4.78,;10.6,-4.01,;11.93,-4.79,;11.93,-6.33,;10.59,-7.09,;9.26,-6.32,;6.6,-1.7,;5.27,-2.47,;5.27,-4,;3.93,-1.69,;3.93,-.15,;5.27,.62,;6.74,.15,;7.63,1.42,;6.71,2.65,;5.25,2.15,;2.6,-2.46,;1.27,-1.7,;1.27,-.16,;-.06,-2.47,;-1.4,-1.7,;-2.73,-2.47,;-4.07,-1.7,;-4.07,-.16,;-5.39,.6,;-6.73,-.17,;-6.73,-1.72,;-5.38,-2.48,;13.27,-2.47,;13.27,-4.02,;14.6,-1.71,;15.94,-2.48,;15.94,-4.02,;17.27,-4.78,;18.52,-3.89,;19.76,-4.8,;19.28,-6.27,;20.04,-7.6,;19.27,-8.93,;17.73,-8.92,;16.96,-7.59,;17.74,-6.26,;17.27,-1.7,;18.61,-2.48,;17.27,-.17,)| Show InChI InChI=1S/C42H51N11O5/c43-38(55)34(22-29-24-48-32-17-8-7-16-31(29)32)52-39(56)33(18-10-20-47-42(44)45)51-40(57)35(21-28-13-5-2-6-14-28)53-41(58)36(23-30-25-46-26-49-30)50-37(54)19-9-15-27-11-3-1-4-12-27/h1-8,11-14,16-17,24-26,33-36,48H,9-10,15,18-23H2,(H2,43,55)(H,46,49)(H,50,54)(H,51,57)(H,52,56)(H,53,58)(H4,44,45,47)/t33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center& Research Institute
Curated by ChEMBL
| Assay Description
Displacement of Eu-NDP-alphaMSH from human MC1 receptor expressed in human HCT116 cells after 1.5 hrs by time-resolved fluorescence analysis |
J Med Chem 54: 8078-84 (2011)
Article DOI: 10.1021/jm201226w
BindingDB Entry DOI: 10.7270/Q2G1618K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
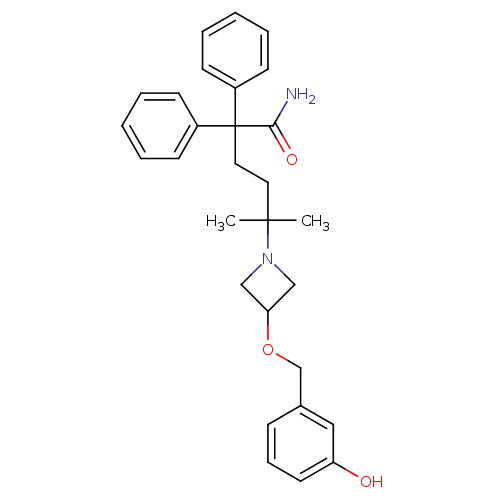
(Homo sapiens (Human)) | BDBM50355623
(CHEMBL1910857)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)OCc1cccc(O)c1 Show InChI InChI=1S/C29H34N2O3/c1-28(2,31-19-26(20-31)34-21-22-10-9-15-25(32)18-22)16-17-29(27(30)33,23-11-5-3-6-12-23)24-13-7-4-8-14-24/h3-15,18,26,32H,16-17,19-21H2,1-2H3,(H2,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
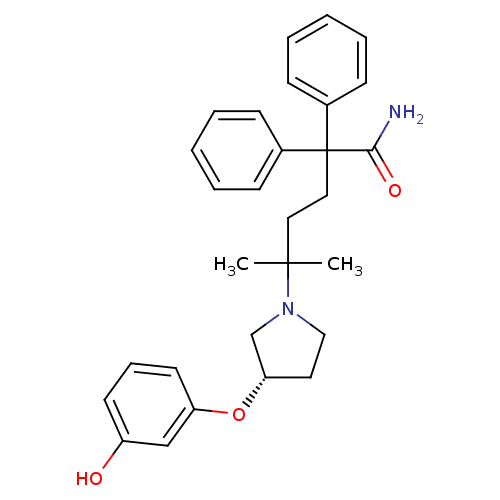
(Homo sapiens (Human)) | BDBM50355617
(CHEMBL1910851)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC[C@@H](C1)Oc1cccc(O)c1 |r| Show InChI InChI=1S/C29H34N2O3/c1-28(2,31-19-16-26(21-31)34-25-15-9-14-24(32)20-25)17-18-29(27(30)33,22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-15,20,26,32H,16-19,21H2,1-2H3,(H2,30,33)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
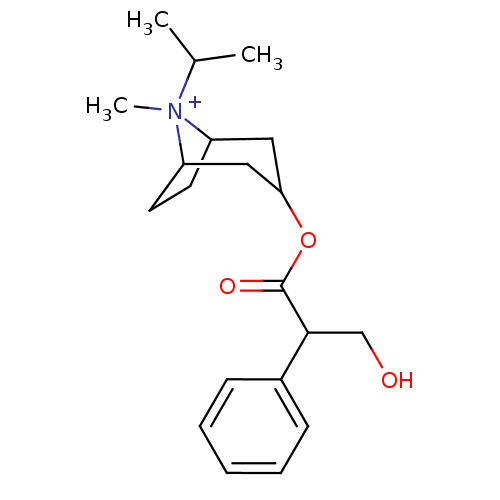
(Homo sapiens (Human)) | BDBM50355610
(CHEMBL1237108)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:6.7:11.9.10,1:3:6.7:11.9.10,(10.09,.76,;9.35,2.05,;7.85,2.05,;10.09,3.35,;11.39,2.6,;10.09,4.89,;10.86,3.55,;10.3,2.58,;8.76,2.58,;7.43,3.35,;7.43,4.89,;8.76,5.66,;6.09,5.66,;6.09,7.2,;7.43,7.97,;4.76,7.97,;4.76,9.51,;3.43,10.28,;3.43,7.2,;3.43,5.66,;2.09,4.89,;.76,5.66,;.76,7.2,;2.09,7.97,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50194659

((2R)-N-(1-cyclopentylmethyl-4-piperidinylmethyl)-1...)Show SMILES O[C@@H]1C[C@H](N(C1)C(=O)CC(c1ccc(F)cc1)(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1CCC[C@@H]1C(=O)NCC1CCN(CC2CCCC2)CC1 Show InChI InChI=1S/C43H51F3N4O4/c44-34-13-7-31(8-14-34)43(32-9-15-35(45)16-10-32,33-11-17-36(46)18-12-33)25-40(52)50-28-37(51)24-39(50)42(54)49-21-3-6-38(49)41(53)47-26-29-19-22-48(23-20-29)27-30-4-1-2-5-30/h7-18,29-30,37-39,51H,1-6,19-28H2,(H,47,53)/t37-,38-,39+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells |
J Med Chem 49: 5653-63 (2006)
Article DOI: 10.1021/jm051205r
BindingDB Entry DOI: 10.7270/Q2F76C6K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50355622

(CHEMBL1910856)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1cccc(O)c1 Show InChI InChI=1S/C28H32N2O3/c1-27(2,30-19-25(20-30)33-24-15-9-14-23(31)18-24)16-17-28(26(29)32,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,18,25,31H,16-17,19-20H2,1-2H3,(H2,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant M3 receptor expressed in CHO-K1 cells assessed as inhibition of carbamoyl choline-induced calcium currents a... |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398820

(CHEMBL2177538)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398820

(CHEMBL2177538)Show InChI InChI=1S/C13H16N2/c1-2-12(9-14-3-1)13-5-10-4-11(6-13)8-15-7-10/h1-3,5,9-11,15H,4,6-8H2/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398850
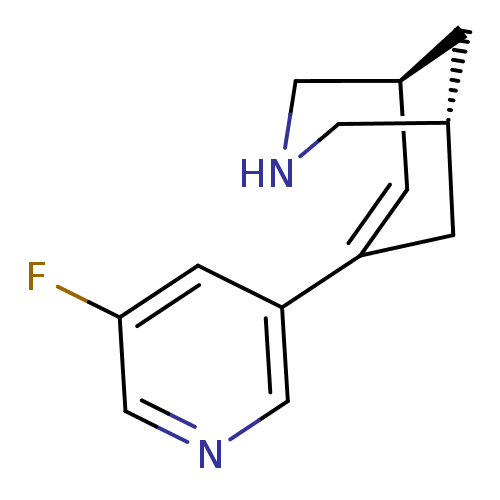
(CHEMBL2177552)Show SMILES Fc1cncc(c1)C1=C[C@H]2CNC[C@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398849
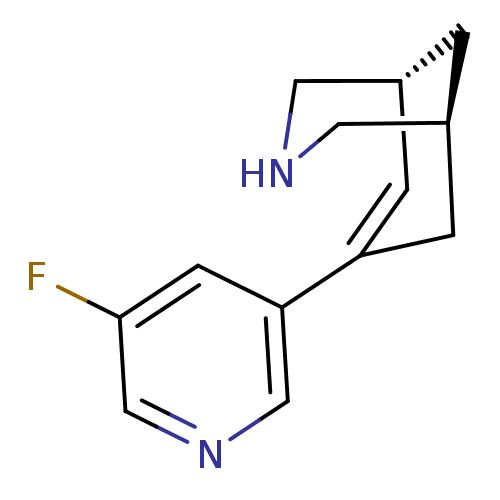
(CHEMBL2177553)Show SMILES Fc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1 |r,t:8| Show InChI InChI=1S/C13H15FN2/c14-13-4-12(7-16-8-13)11-2-9-1-10(3-11)6-15-5-9/h2,4,7-10,15H,1,3,5-6H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398832
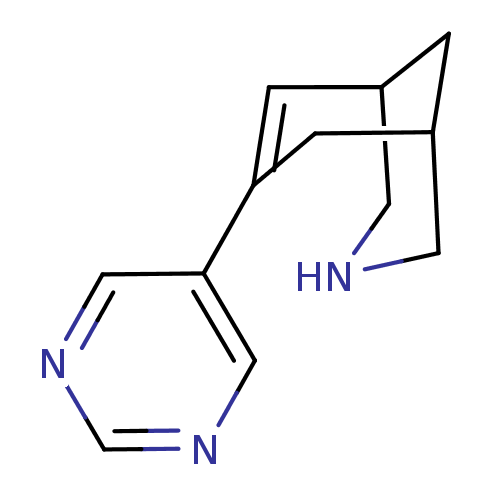
(CHEMBL2177518)Show SMILES C1NCC2CC1CC(=C2)c1cncnc1 |c:8,TLB:9:7:0.1.2:4| Show InChI InChI=1S/C12H15N3/c1-9-2-11(3-10(1)5-13-4-9)12-6-14-8-15-7-12/h2,6-10,13H,1,3-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells |
J Med Chem 55: 9929-45 (2012)
Article DOI: 10.1021/jm3011299
BindingDB Entry DOI: 10.7270/Q2474C0B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data