

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132351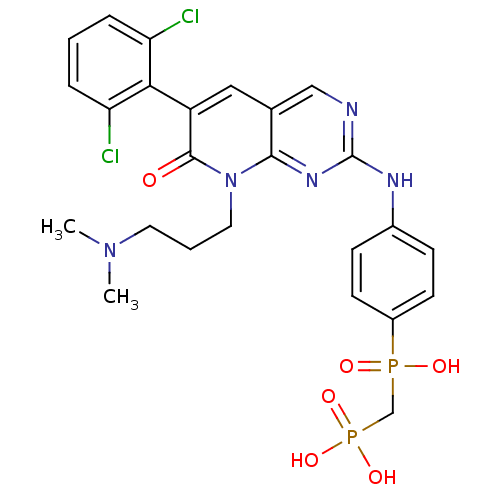 (({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM13930 ((2S)-N-tert-butyl-1-[(2R)-2-[(8S,11S)-8-(carbamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 37: 215-8 (1994) Article DOI: 10.1021/jm00028a001 BindingDB Entry DOI: 10.7270/Q2DR2SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM902 ((3R)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 37: 215-8 (1994) Article DOI: 10.1021/jm00028a001 BindingDB Entry DOI: 10.7270/Q2DR2SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132348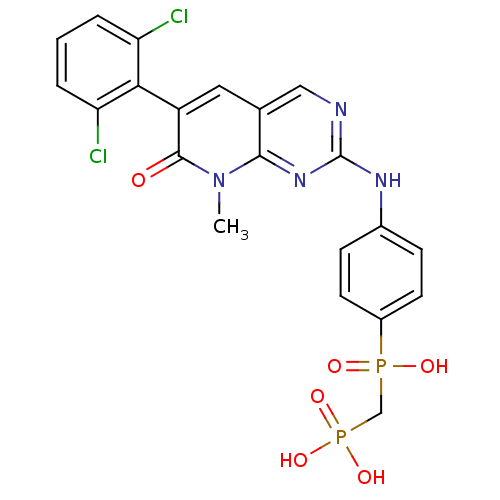 (({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132322 (({2-[(2-{4-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2 (CDK2) | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132554 ((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132550 ((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-propionyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132541 ((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132541 ((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM13931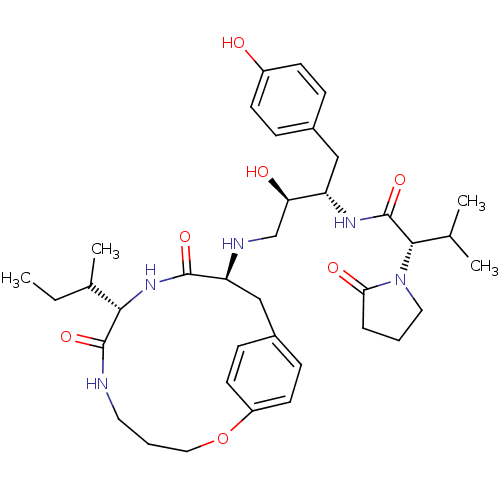 ((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2R)-butan-2-yl]-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 37: 215-8 (1994) Article DOI: 10.1021/jm00028a001 BindingDB Entry DOI: 10.7270/Q2DR2SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132558 ((E)-(12S,13R,14S,17R,21S,23S,24R,25R,27R)-17-Ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132542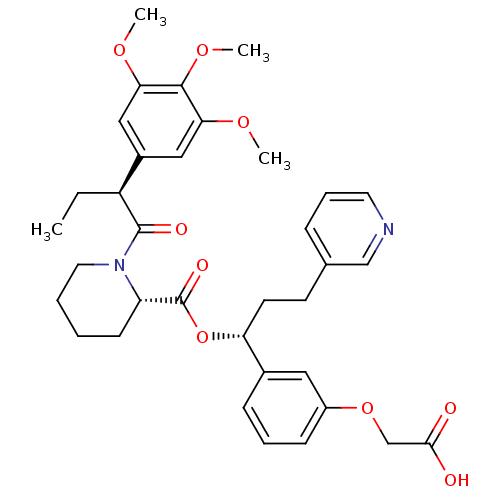 ((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3071 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 38 | 2-ani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221656 (CHEMBL3706663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132560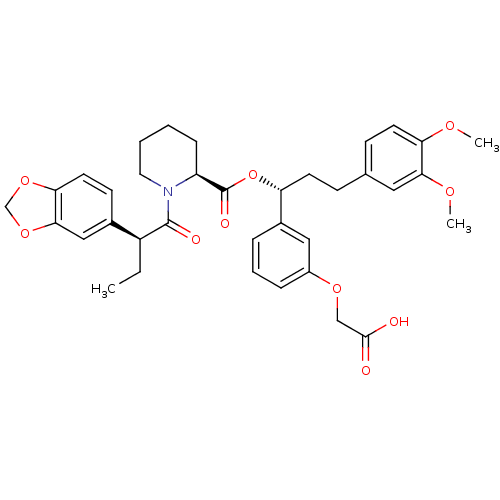 ((S)-1-((S)-2-Benzo[1,3]dioxol-5-yl-butyryl)-piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132324 (3-{4-Amino-7-[4-(2-hydroxy-ethyl)-phenyl]-7H-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132339 (3-[4-Amino-7-(4-{2-[(2-hydroxy-ethyl)-methyl-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132544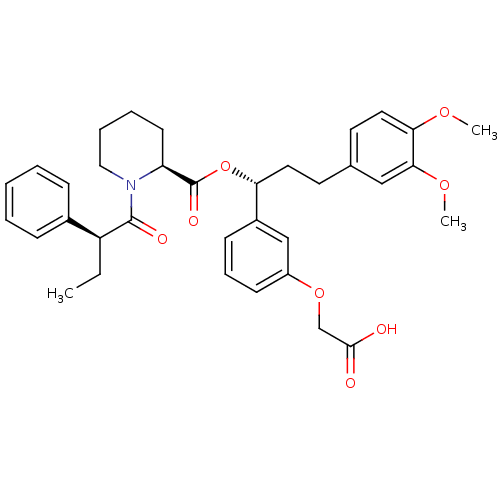 ((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132544 ((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451556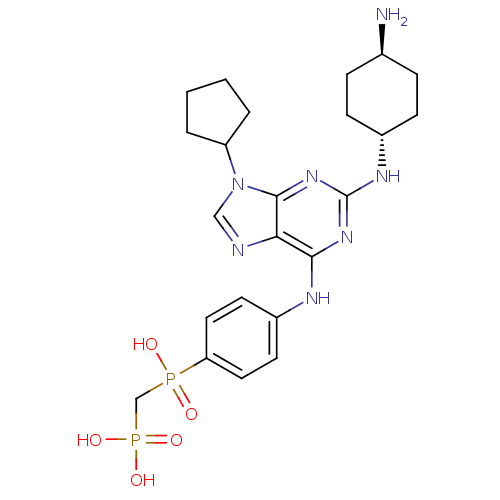 (CHEMBL3084838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132556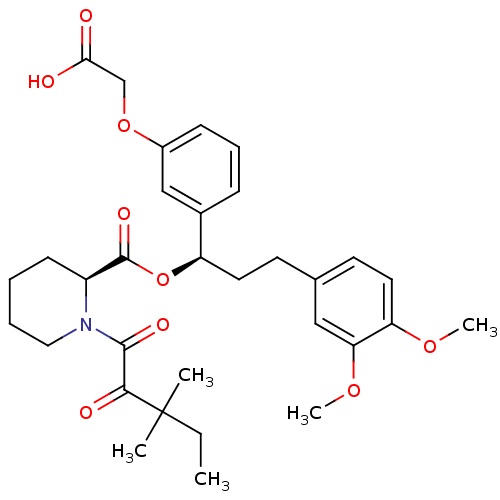 ((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132334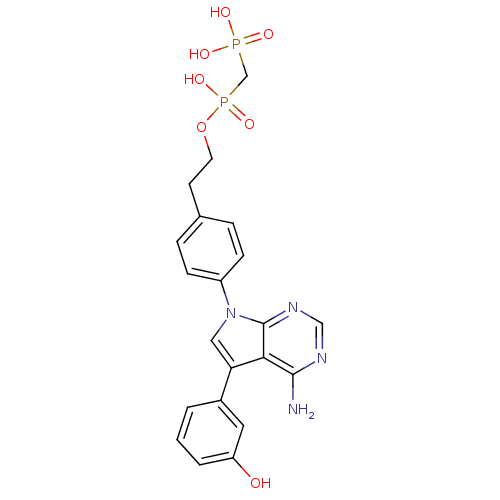 (CHEMBL104215 | [(2-{4-[4-Amino-5-(3-hydroxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132551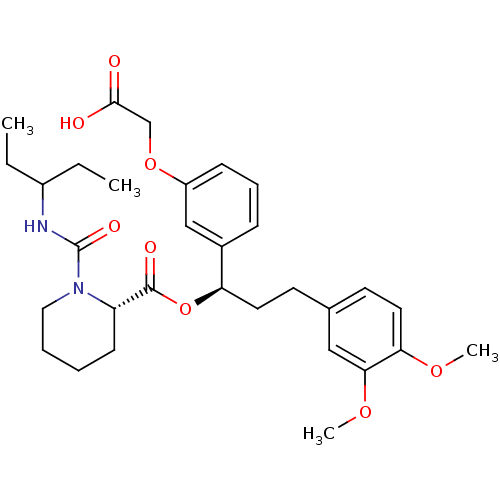 (1-(1-Ethyl-propylcarbamoyl)-piperidine-2-carboxyli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 | Bioorg Med Chem Lett 13: 3181-4 (2003) BindingDB Entry DOI: 10.7270/Q2BV7G0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132352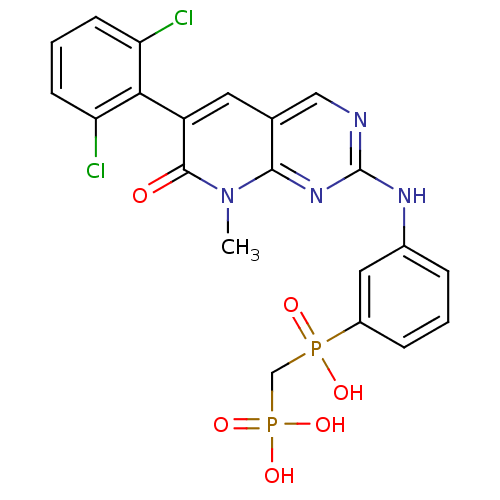 (({3-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372174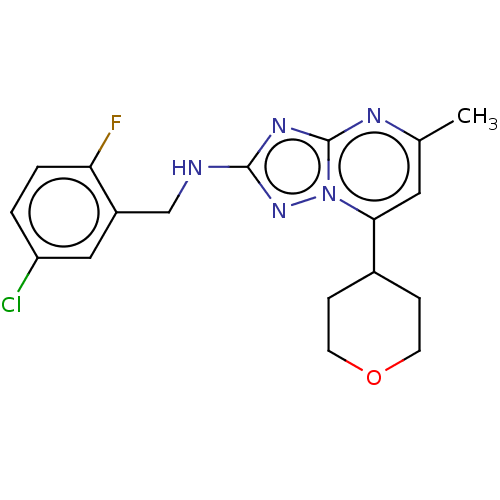 (US10239882, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372175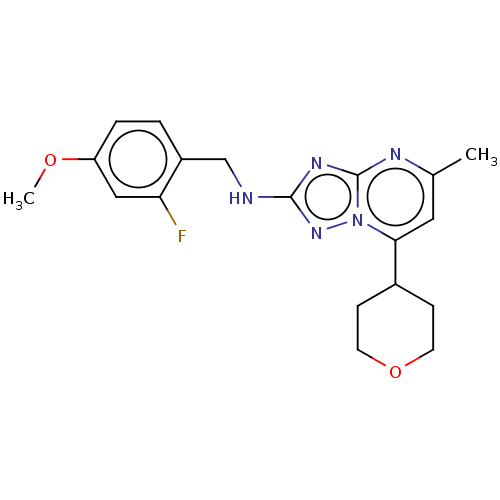 (US10239882, Example 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372176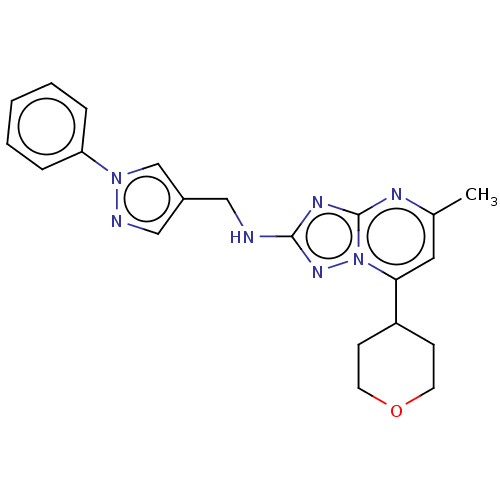 (US10239882, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372177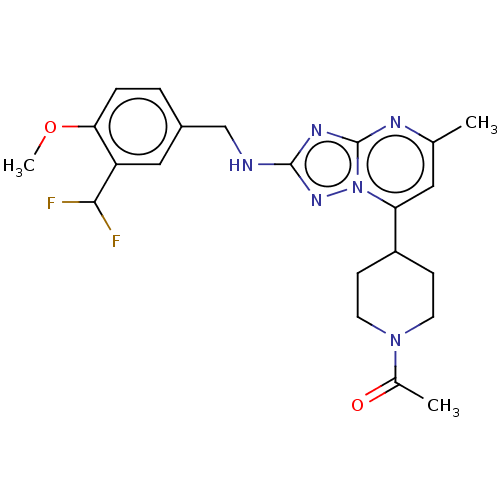 (US10239882, Example 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372178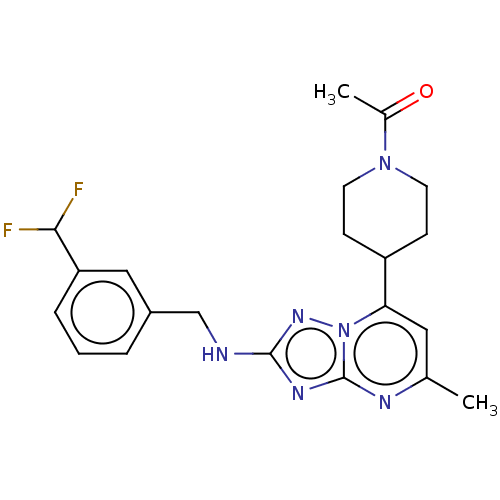 (US10239882, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372179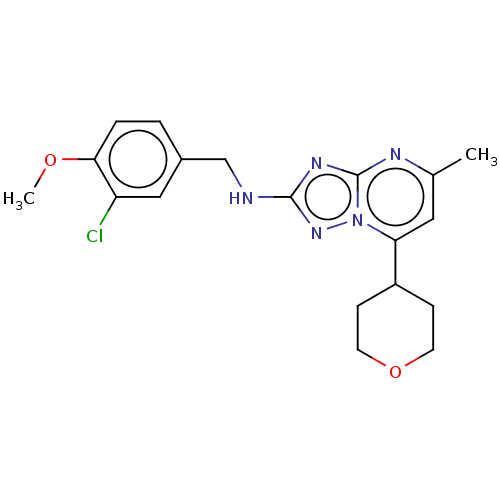 (US10239882, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372180 (US10239882, Example 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372181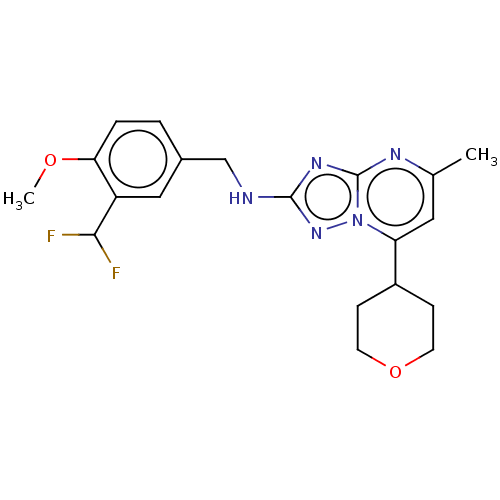 (US10239882, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372182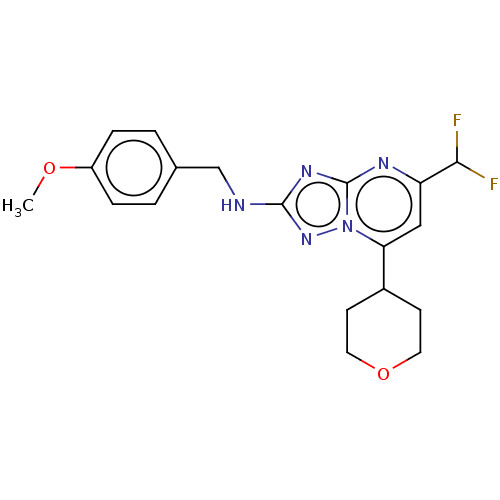 (US10239882, Example 29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372183 (US10239882, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372184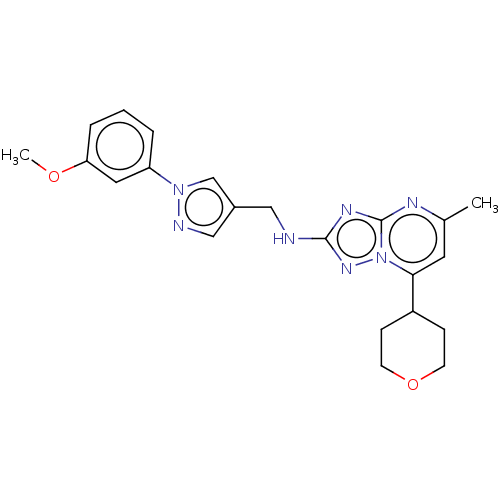 (US10239882, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372185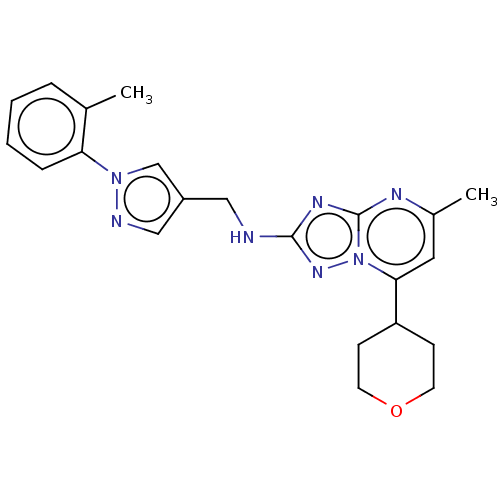 (US10239882, Example 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372186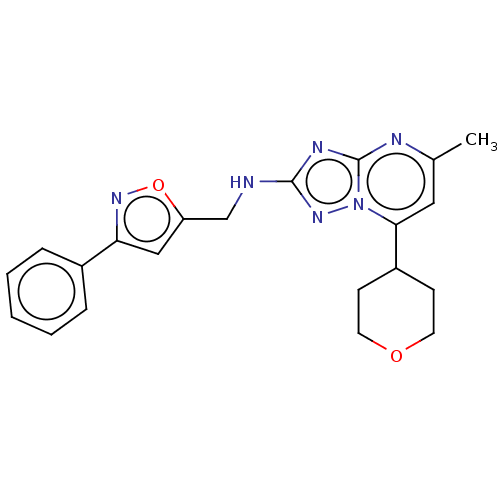 (US10239882, Example 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372187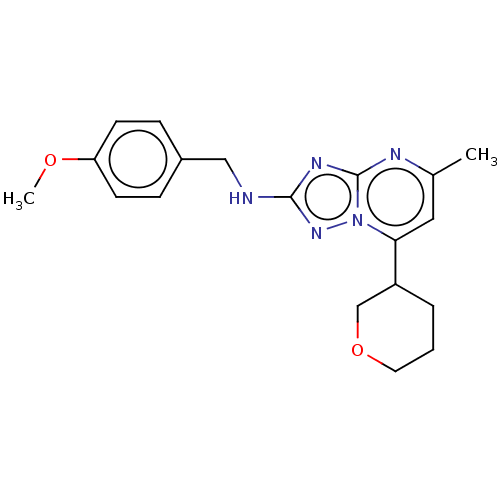 (US10239882, Example 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372188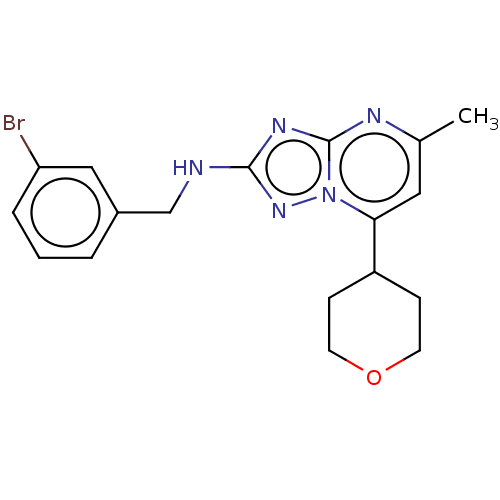 (US10239882, Example 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372189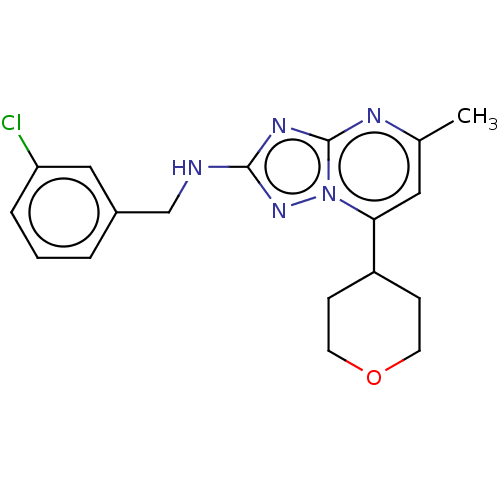 (US10239882, Example 40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372190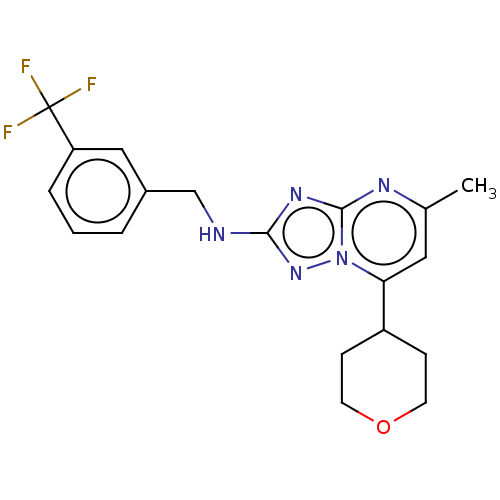 (US10239882, Example 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372191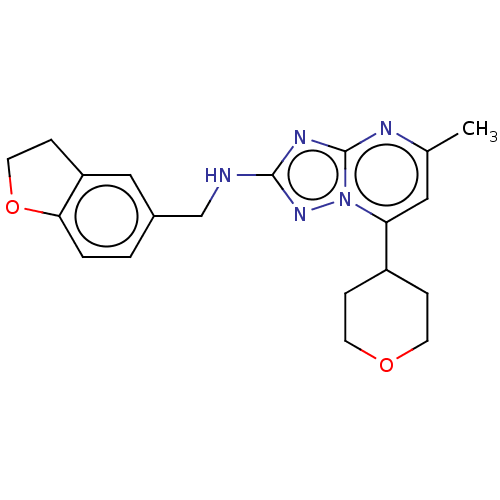 (US10239882, Example 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372192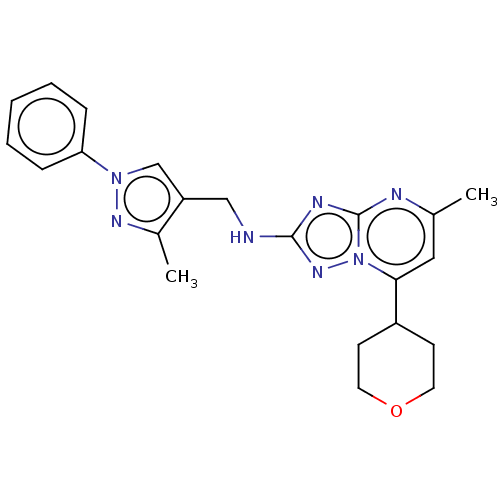 (US10239882, Example 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372193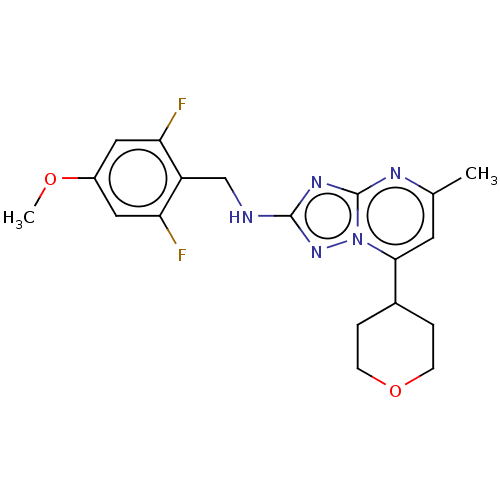 (US10239882, Example 50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372194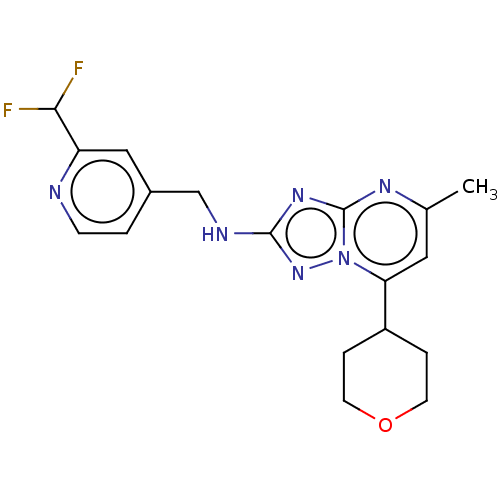 (US10239882, Example 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372195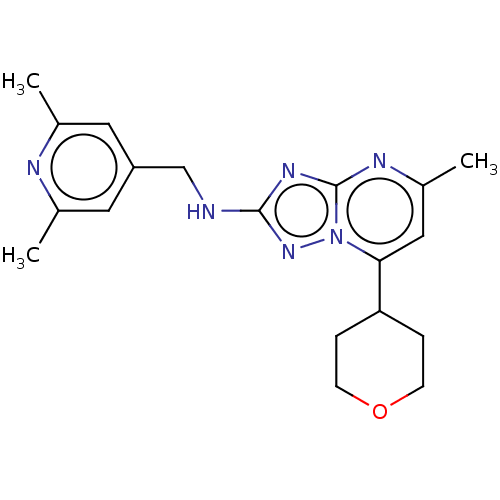 (US10239882, Example 53) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372196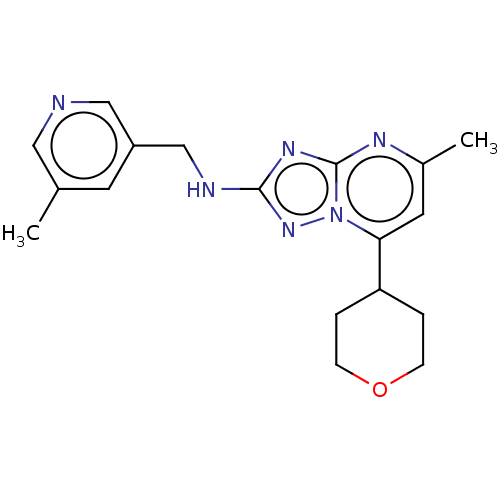 (US10239882, Example 54) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372197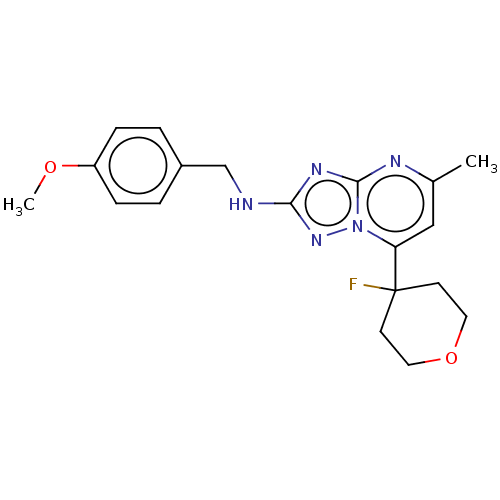 (US10239882, Example 55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM372198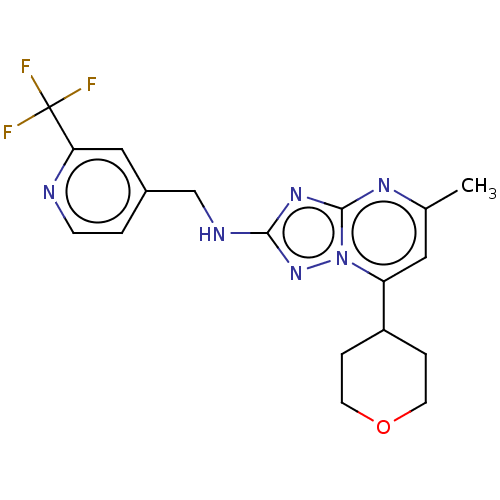 (US10239882, Example 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | J Med Chem 51: 2795-806 (2008) BindingDB Entry DOI: 10.7270/Q24J0HFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |