

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099016 (CHEMBL275964 | Cyclohexanecarboxylic acid {(S)-1-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166904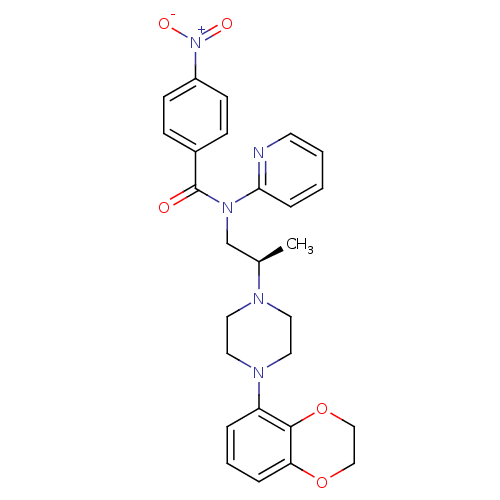 (CHEMBL193206 | N-{(R)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166901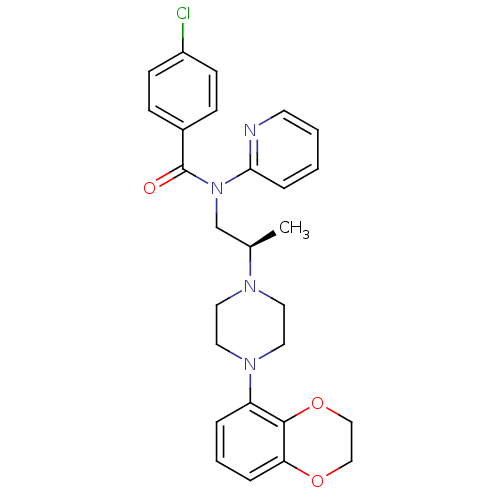 (4-Chloro-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099015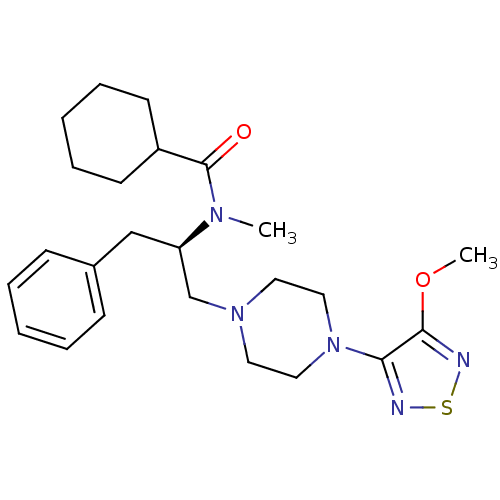 (CHEMBL10796 | Cyclohexanecarboxylic acid {(R)-1-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166903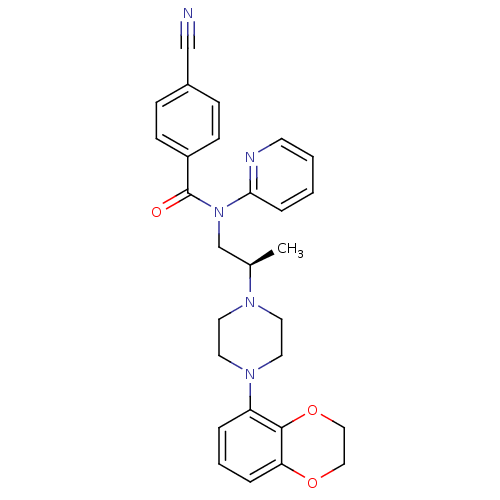 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166899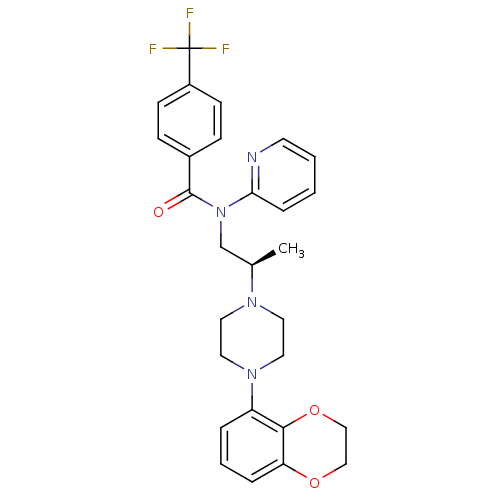 (CHEMBL435111 | N-{(R)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099011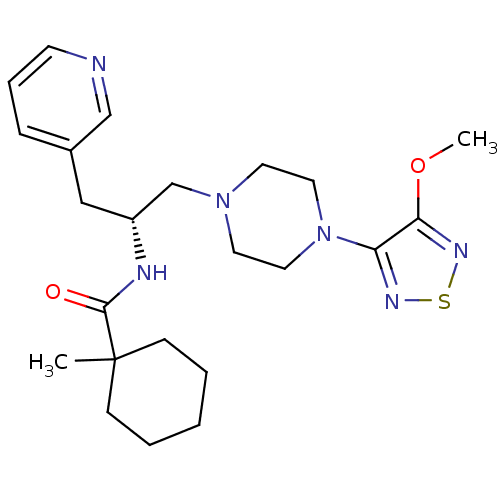 (1-Methyl-cyclohexanecarboxylic acid {(R)-2-[4-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166902 (CHEMBL195425 | N-{(S)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099014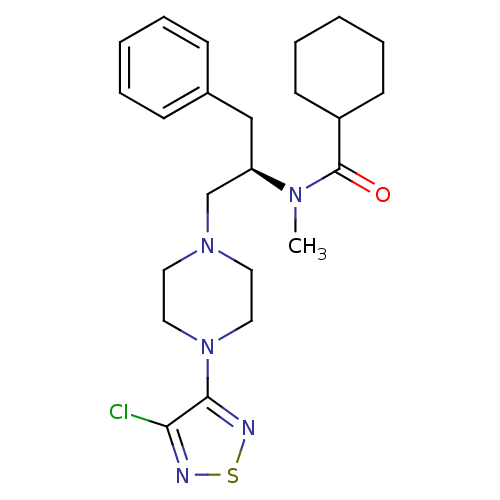 (CHEMBL10793 | Cyclohexanecarboxylic acid {(R)-1-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166900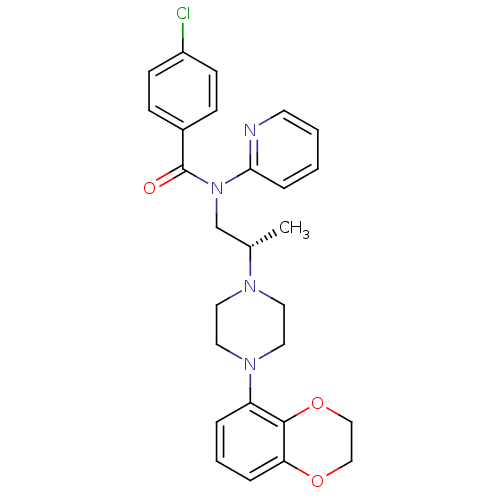 (4-Chloro-N-{(S)-2-[4-(2,3-dihydro-benzo[1,4]dioxin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166906 (CHEMBL365350 | N-{(S)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166905 (4-Cyano-N-{(S)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099013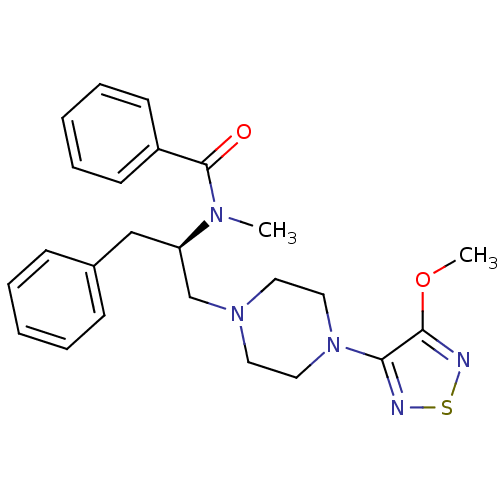 (CHEMBL10603 | N-{(R)-1-Benzyl-2-[4-(4-methoxy-[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211726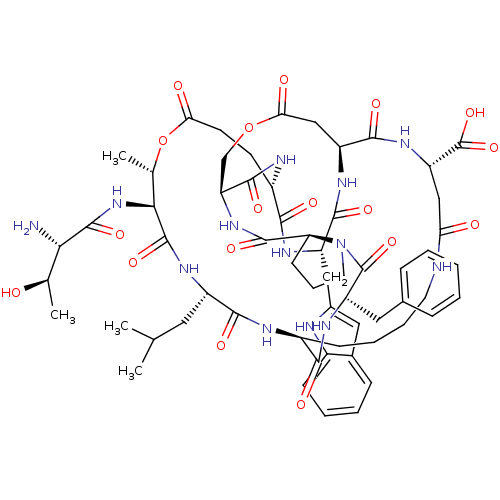 (CHEMBL403896 | FR-901451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50080264 (1N-[21-benzyl-29-[(Z)-ethylidene]-13,14,23-trihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211725 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211723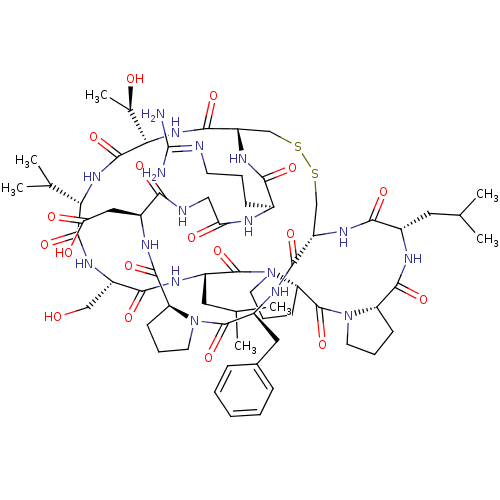 (2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211721 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50211722 ((2S)-2-{[(3S,9S,12S,15S,18S,21R,26R,29S,32S)-21-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase | Bioorg Med Chem 15: 4618-28 (2007) Article DOI: 10.1016/j.bmc.2007.03.082 BindingDB Entry DOI: 10.7270/Q2D21ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50099012 (CHEMBL10764 | N-{(S)-1-Benzyl-2-[4-(4-chloro-[1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 11: 1069-71 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50223719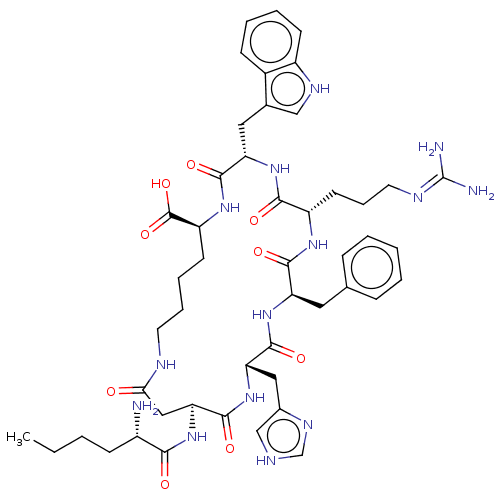 (CHEMBL3348530) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Intracellular level of cAMP in cells expressing the melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849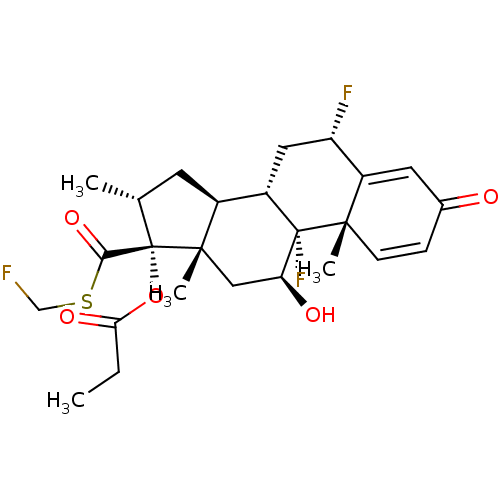 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50138293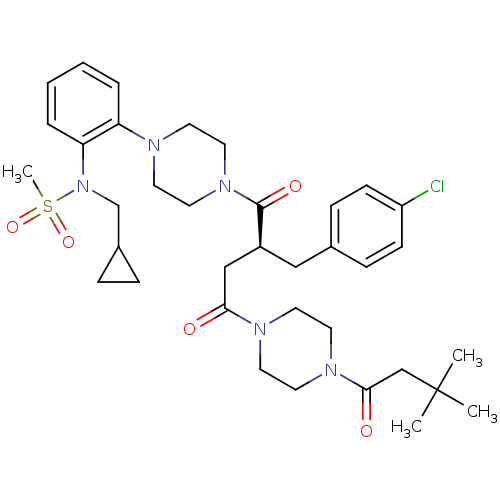 ((S)-2-(4-Chloro-benzyl)-1-{4-[2-(2-cyclopropyl-1-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Intracellular level of cAMP in cells expressing the melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20464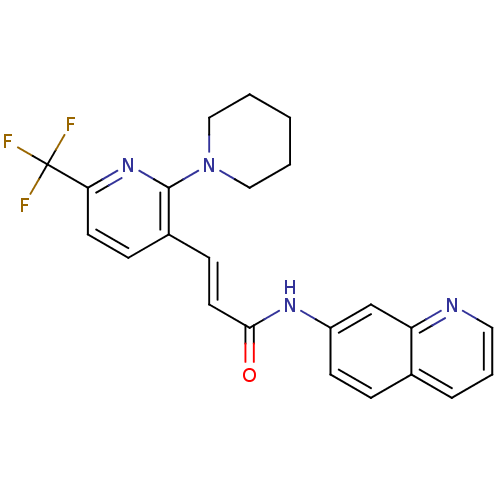 ((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of capsaicin-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357083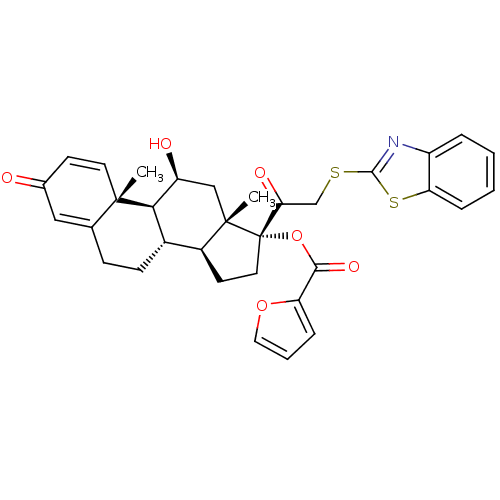 (CHEMBL1917414) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357084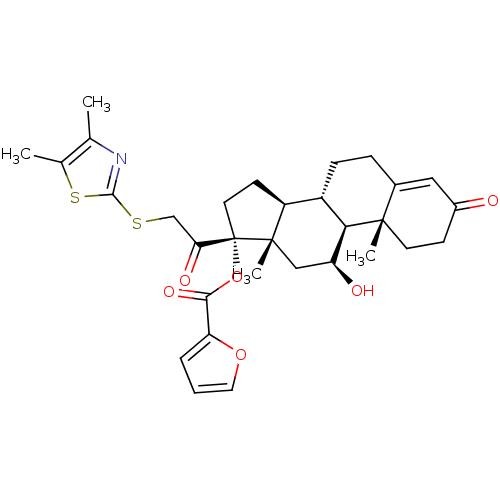 (CHEMBL1917413) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20464 ((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of pH-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50158665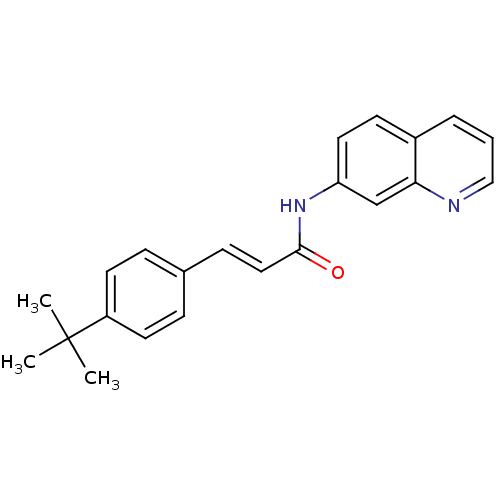 ((E)-3-(4-tert-Butyl-phenyl)-N-quinolin-7-yl-acryla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of capsaicin-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50237710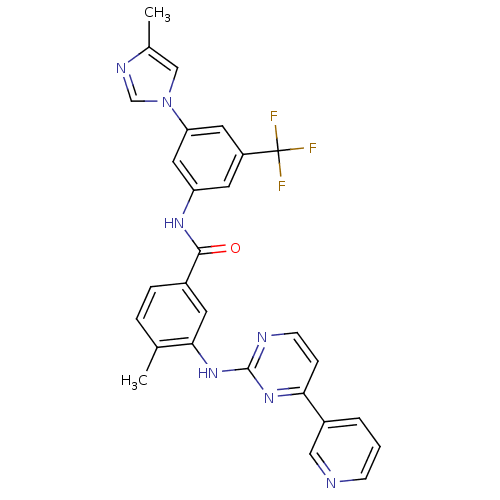 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nyrada Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged Abl (2 to 1130 residues) expressed in baculovirus expression system using FAM-labelled P2 pepti... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115344 BindingDB Entry DOI: 10.7270/Q2Z89H0P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50158658 ((E)-3-(2-Morpholin-4-yl-6-trifluoromethyl-pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of pH-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50223719 (CHEMBL3348530) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-NDP-alpha-MSH binding to the human melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50138288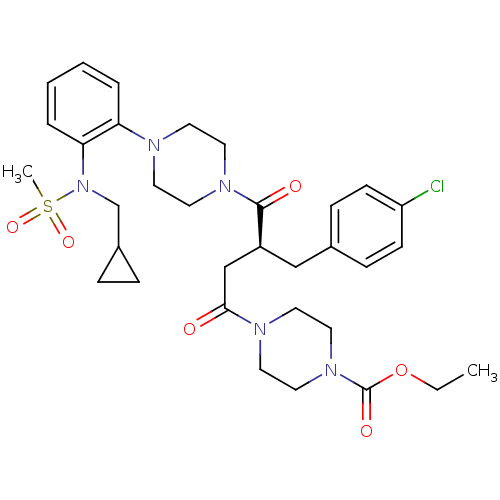 (4-((S)-3-(4-Chloro-benzyl)-4-{4-[2-(2-cyclopropyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-NDP-alpha-MSH binding to the human melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357093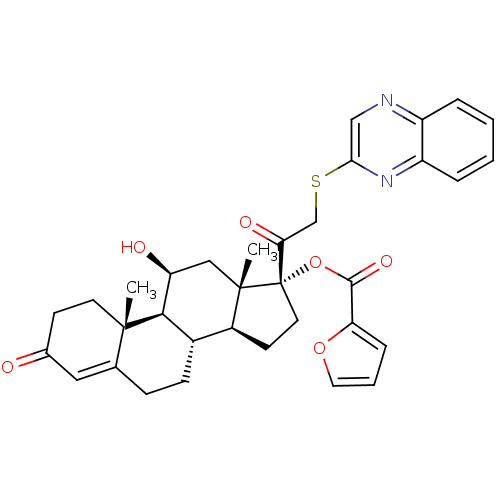 (CHEMBL1917251) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50362450 (CHEMBL1940557) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-8 production | Bioorg Med Chem Lett 22: 1086-90 (2012) Article DOI: 10.1016/j.bmcl.2011.11.120 BindingDB Entry DOI: 10.7270/Q2JH3MNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50362458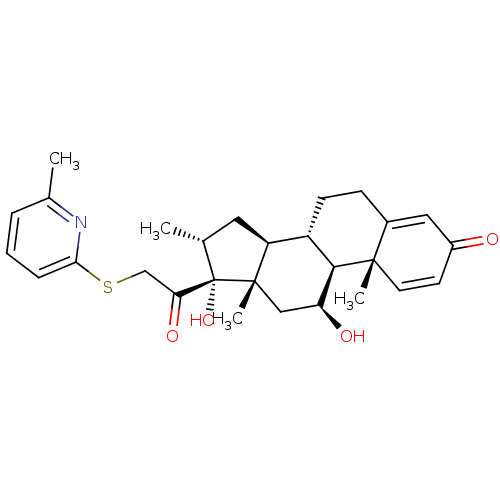 (CHEMBL1940694) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-8 production | Bioorg Med Chem Lett 22: 1086-90 (2012) Article DOI: 10.1016/j.bmcl.2011.11.120 BindingDB Entry DOI: 10.7270/Q2JH3MNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50158658 ((E)-3-(2-Morpholin-4-yl-6-trifluoromethyl-pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of capsaicin-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357086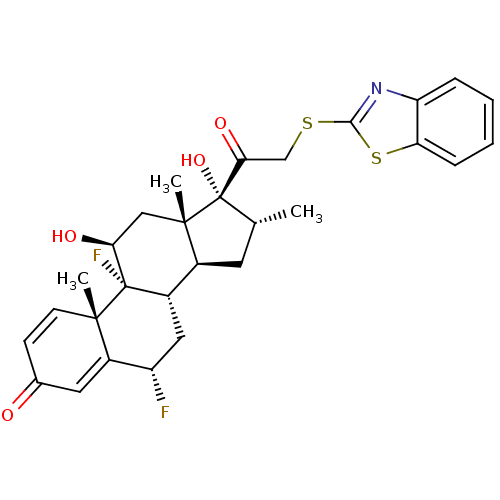 (CHEMBL1917246) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357091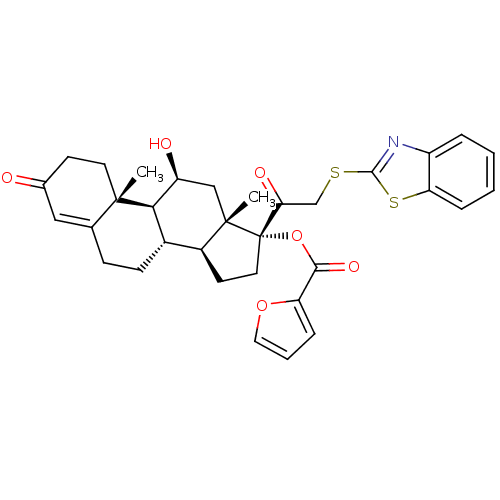 (CHEMBL1917254) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50158665 ((E)-3-(4-tert-Butyl-phenyl)-N-quinolin-7-yl-acryla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of pH-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50138301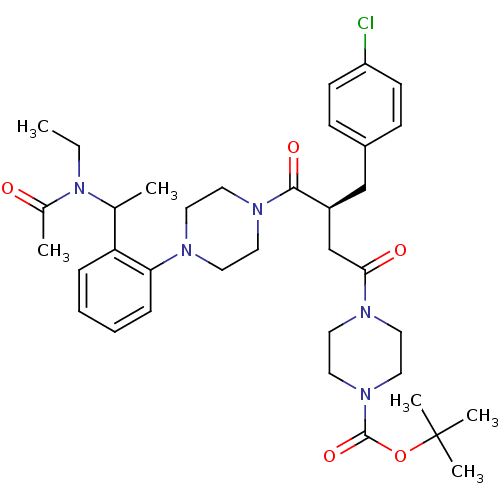 (4-[(S)-4-(4-{2-[1-(Acetyl-ethyl-amino)-ethyl]-phen...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-NDP-alpha-MSH binding to the human melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357087 (CHEMBL1917416) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357088 (CHEMBL1917415) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357085 (CHEMBL1917253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50158681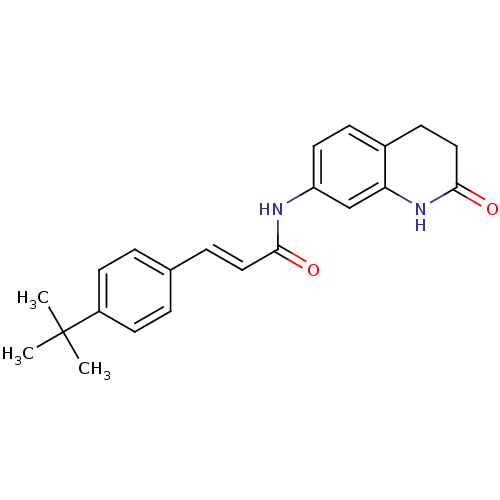 ((E)-3-(4-tert-Butyl-phenyl)-N-(2-oxo-1,2,3,4-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of capsaicin-induced calcium uptake by transient receptor potential vanilloid type 1 expressed in CHO cells | J Med Chem 48: 71-90 (2005) Article DOI: 10.1021/jm049485i BindingDB Entry DOI: 10.7270/Q2J67GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50357094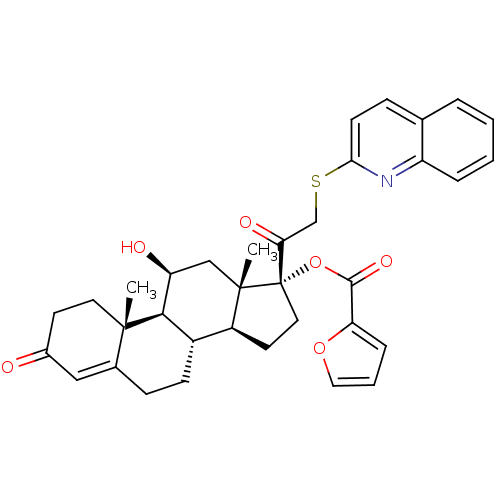 (CHEMBL1917250) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-stimulated IL-6 cytokine synthesis | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50130009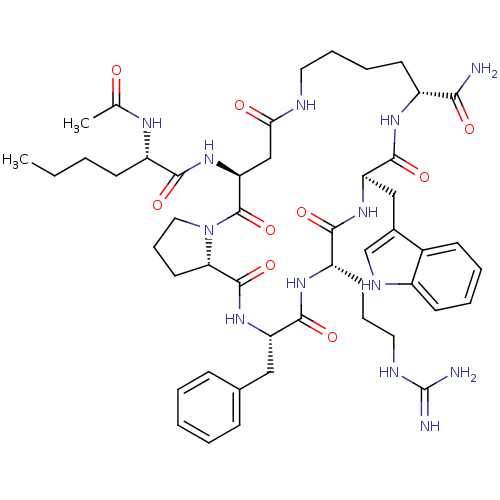 ((4S,15R,18S,21S,24S)-5-((S)-2-Acetylamino-hexanoyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor (hMC4R) using [125I]NDP-alpha-MSH as radioligand | Bioorg Med Chem Lett 13: 2337-40 (2003) BindingDB Entry DOI: 10.7270/Q21G0MTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50138290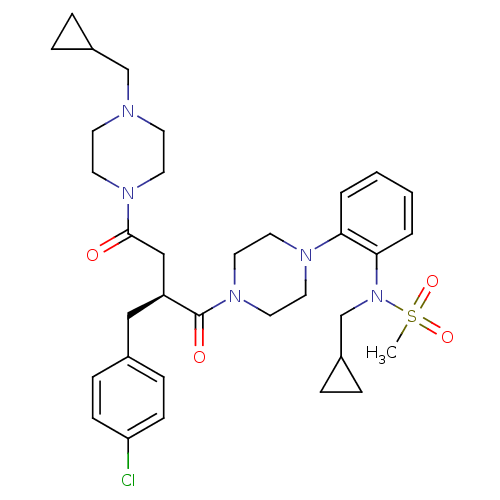 ((S)-2-(4-Chloro-benzyl)-1-{4-[2-(2-cyclopropyl-1-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-NDP-alpha-MSH binding to the human melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50138294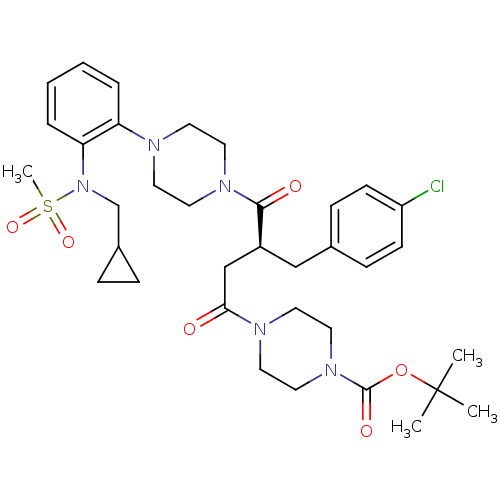 (4-((S)-3-(4-Chloro-benzyl)-4-{4-[2-(2-cyclopropyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-NDP-alpha-MSH binding to the human melanocortin 4 receptor | Bioorg Med Chem Lett 14: 377-81 (2003) BindingDB Entry DOI: 10.7270/Q2902363 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 623 total ) | Next | Last >> |