

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50128319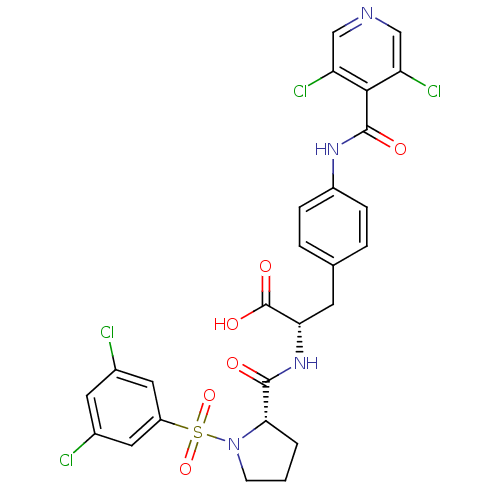 ((S)-2-{[(S)-1-(3,5-Dichloro-benzenesulfonyl)-pyrro...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258532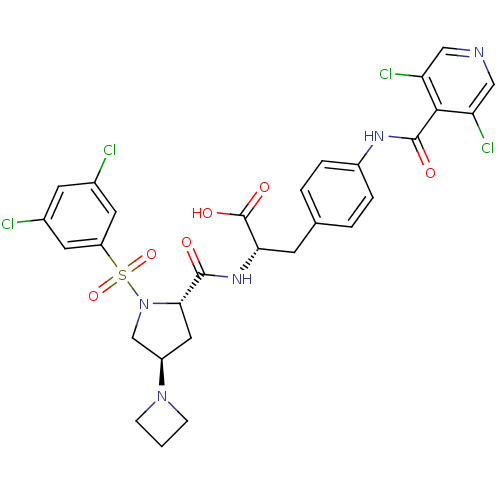 (CHEMBL502641 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258535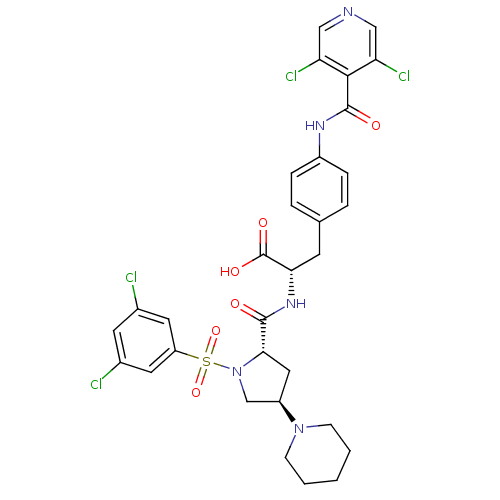 (CHEMBL448863 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258531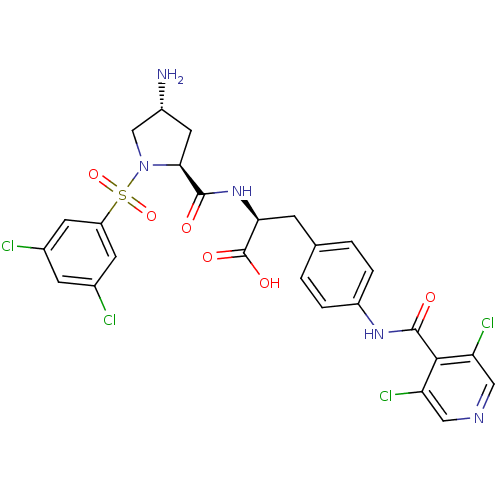 (CHEMBL507665 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258536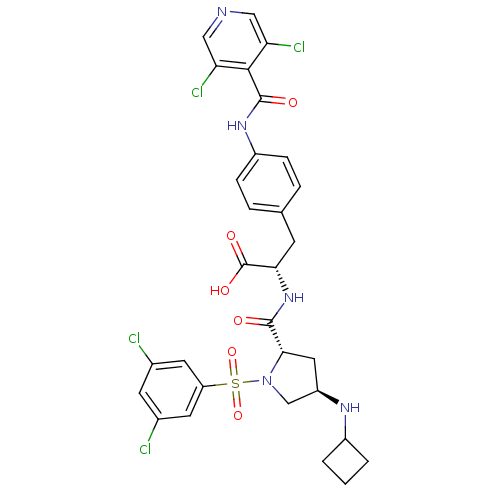 (CHEMBL445303 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258534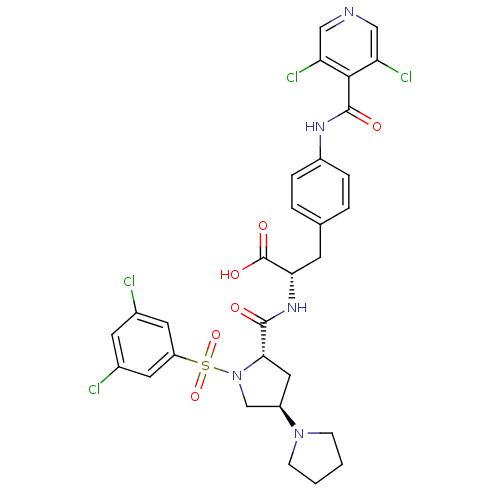 (CHEMBL505992 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258536 (CHEMBL445303 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258530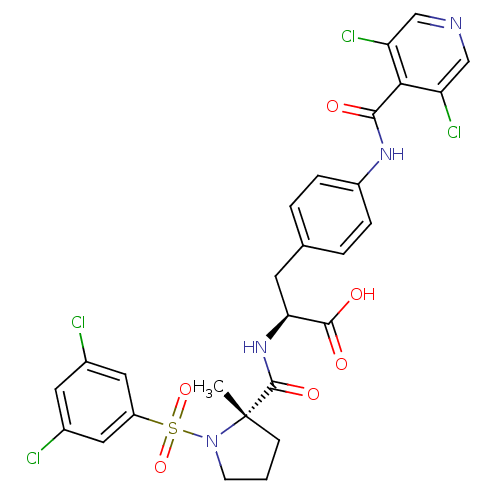 ((S)-3-(4-(3,5-dichloroisonicotinamido)phenyl)-2-((...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258533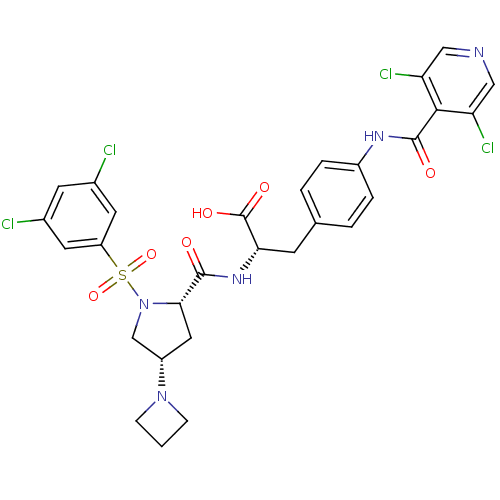 (CHEMBL444822 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258532 (CHEMBL502641 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258534 (CHEMBL505992 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104550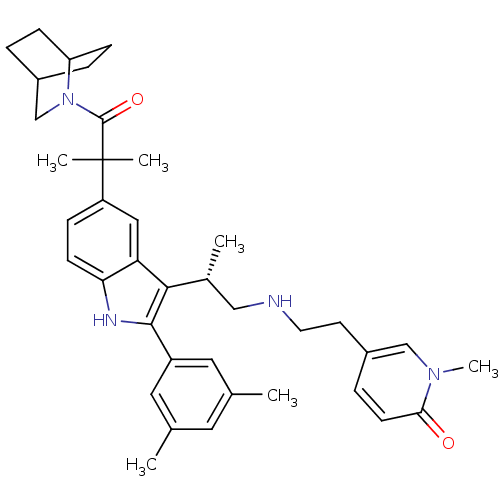 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385814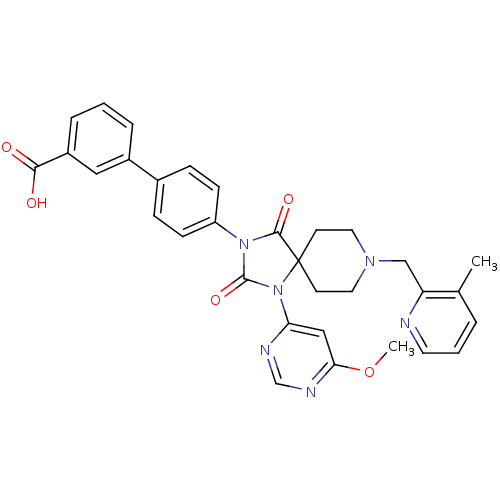 (CHEMBL2043169) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385806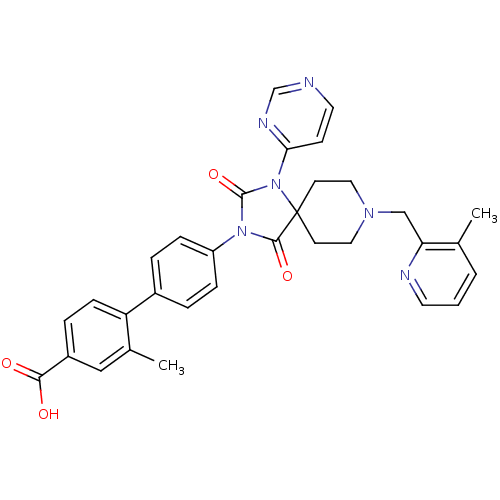 (CHEMBL2041193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385805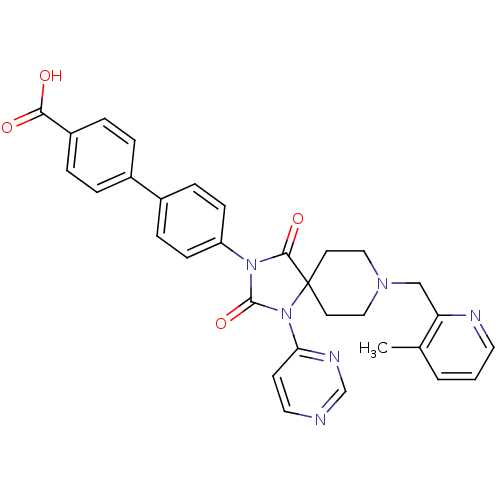 (CHEMBL2041192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385803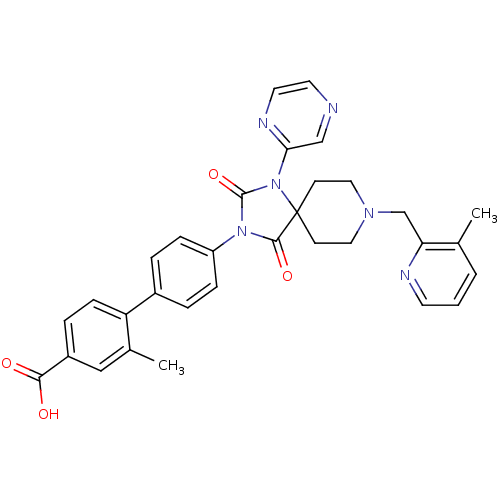 (CHEMBL2041190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385799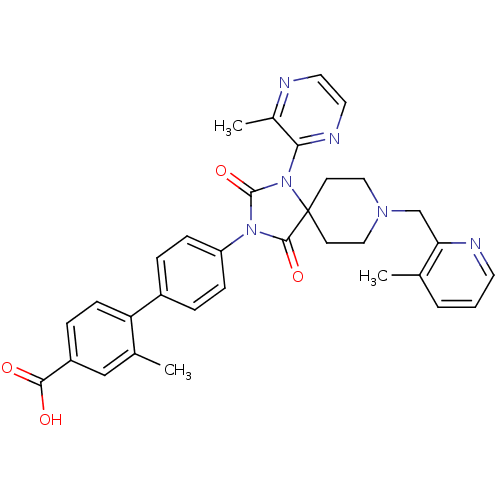 (CHEMBL2041186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385788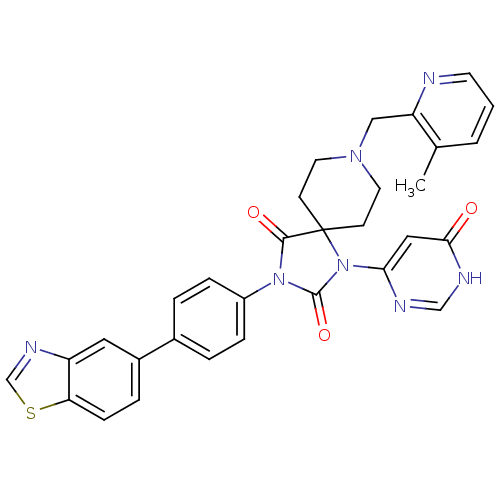 (CHEMBL2041175) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385796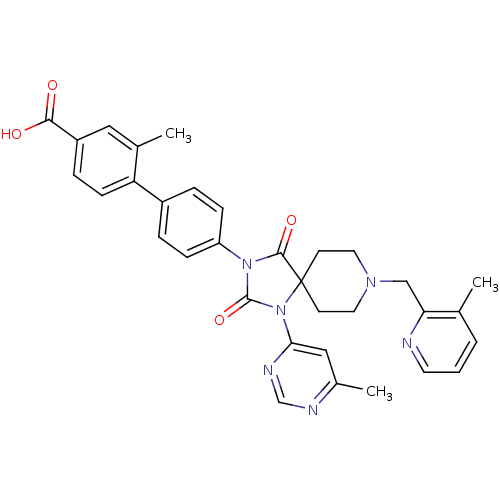 (CHEMBL2041182) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385830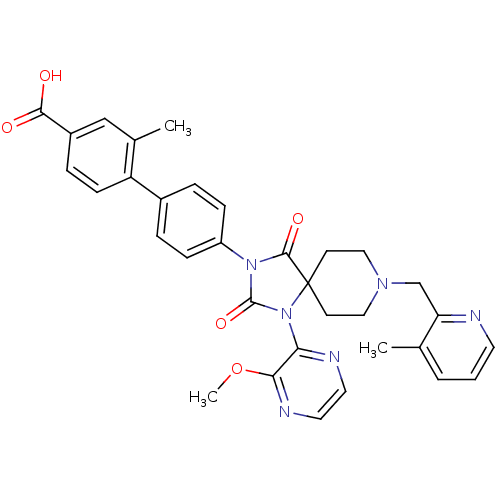 (CHEMBL2041185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385782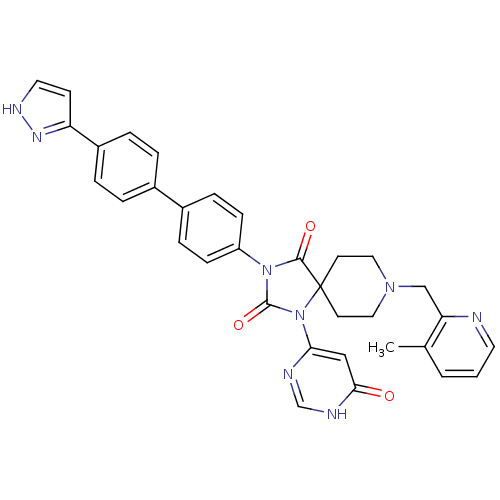 (CHEMBL2041169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385819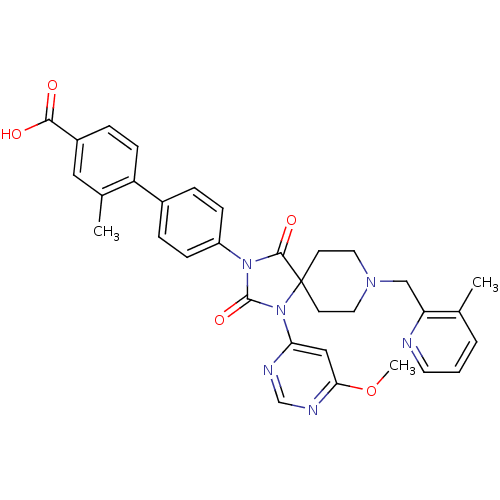 (CHEMBL2043325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385806 (CHEMBL2041193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385803 (CHEMBL2041190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385830 (CHEMBL2041185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385796 (CHEMBL2041182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385782 (CHEMBL2041169) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385819 (CHEMBL2043325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385798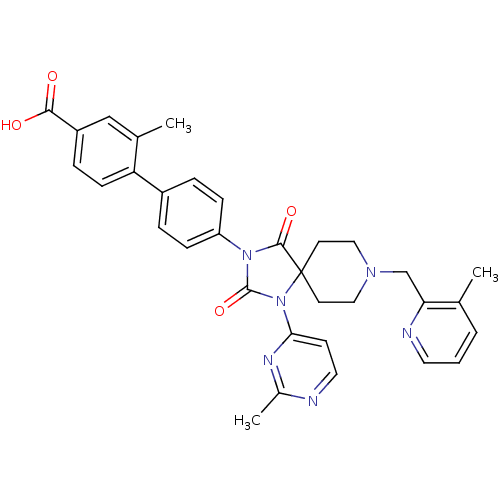 (CHEMBL2041184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385788 (CHEMBL2041175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385799 (CHEMBL2041186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385826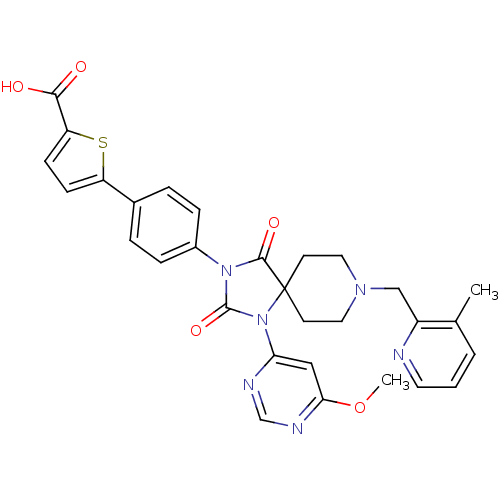 (CHEMBL2041010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385805 (CHEMBL2041192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385798 (CHEMBL2041184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104554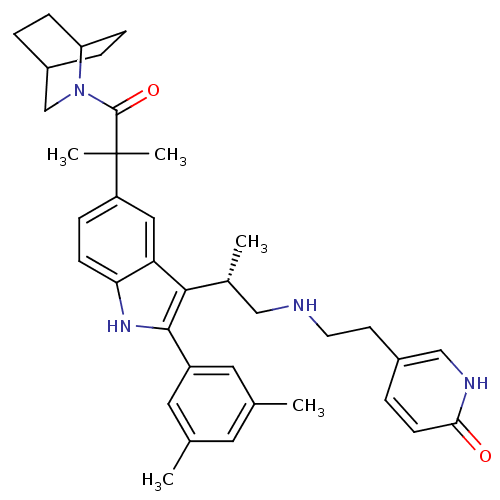 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional antagonism via inhibition of GnRH-stimulated phosphatidylinositol (PI) hydrolysis in cloned Chinese hamster ovary (CHO) cells sta... | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385801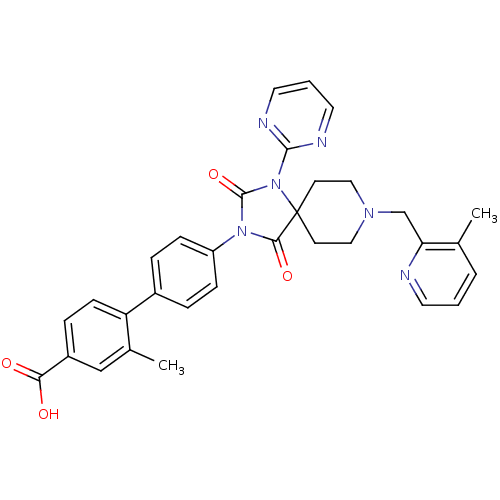 (CHEMBL2041188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385820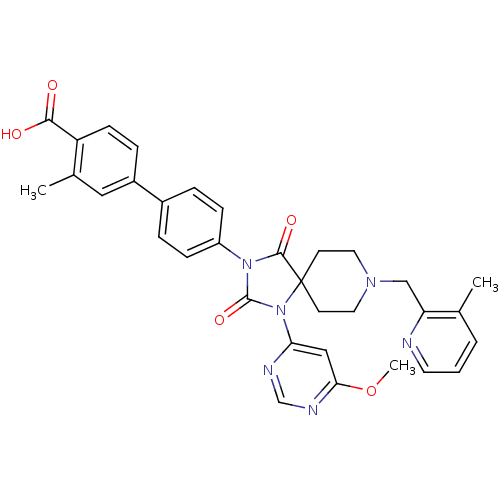 (CHEMBL2043326) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385813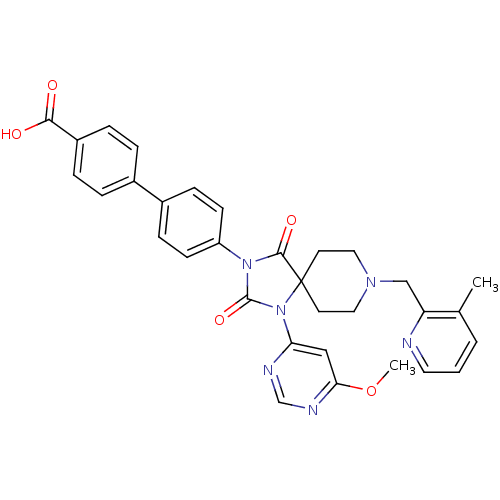 (CHEMBL2043168) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258535 (CHEMBL448863 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385791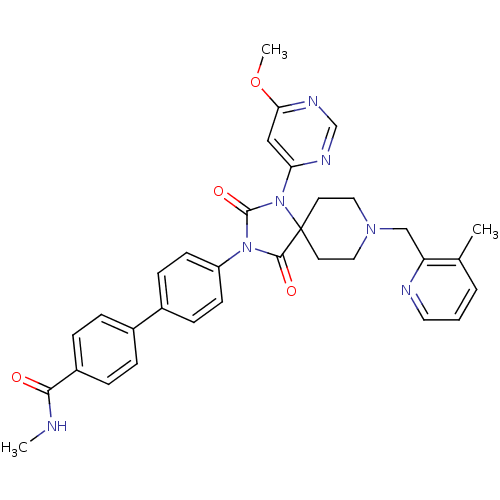 (CHEMBL2041178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385801 (CHEMBL2041188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385802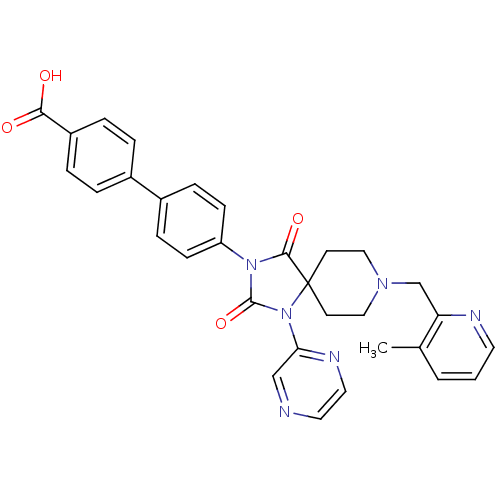 (CHEMBL2041189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104549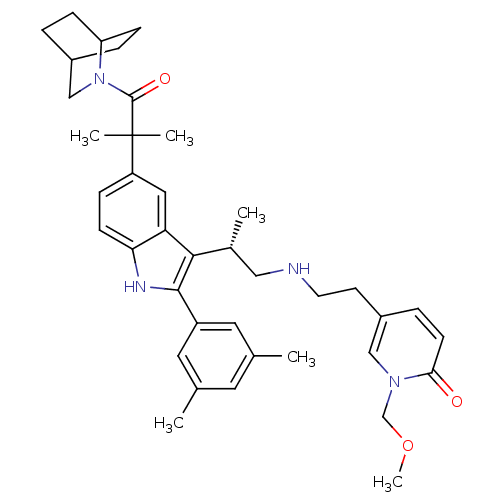 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258531 (CHEMBL507665 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104551 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104564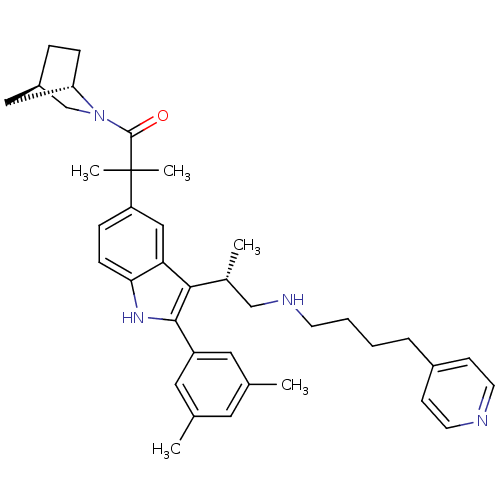 (1-(1S,4R)-2-Aza-bicyclo[2.2.1]hept-2-yl-2-{2-(3,5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50385811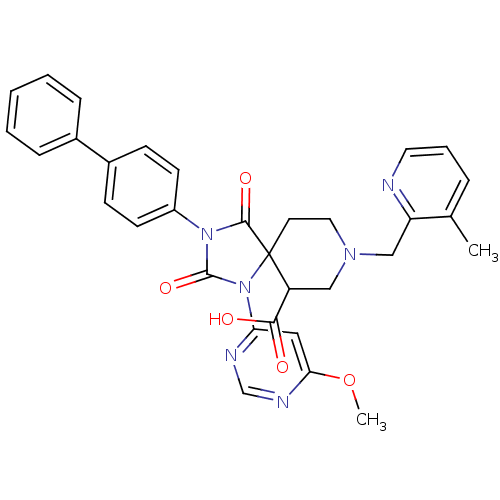 (CHEMBL2043010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged PHD2 expressed in baculovirus infected insect sf9 cells using biotinyl-DLDLEMLAPYIPMDDDFQL as substrate preincubated with c... | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385818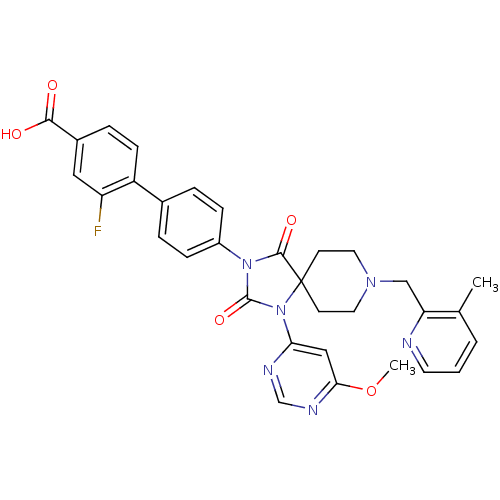 (CHEMBL2043324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385825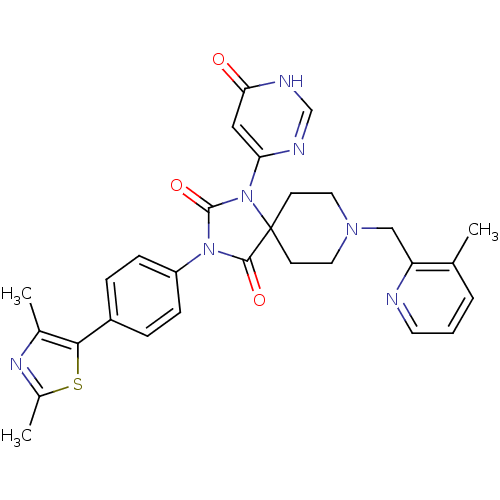 (CHEMBL2040897) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50385797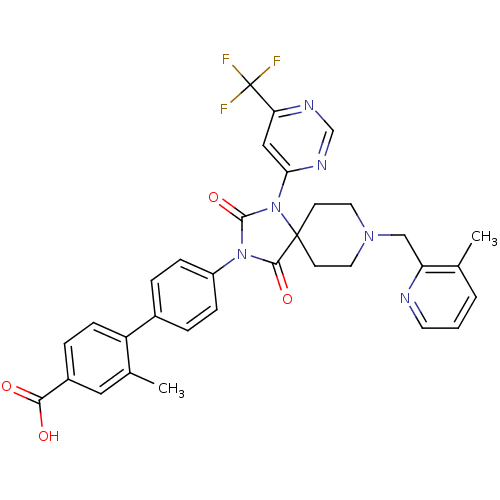 (CHEMBL2041183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PHD1 | J Med Chem 55: 2945-59 (2012) Article DOI: 10.1021/jm201542d BindingDB Entry DOI: 10.7270/Q27945QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 517 total ) | Next | Last >> |