

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50392999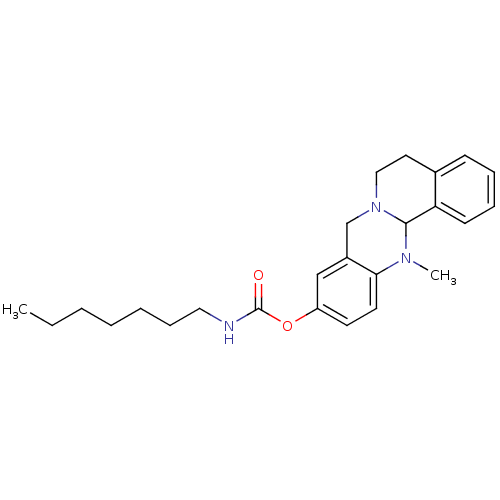 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of Electric eel AChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50392998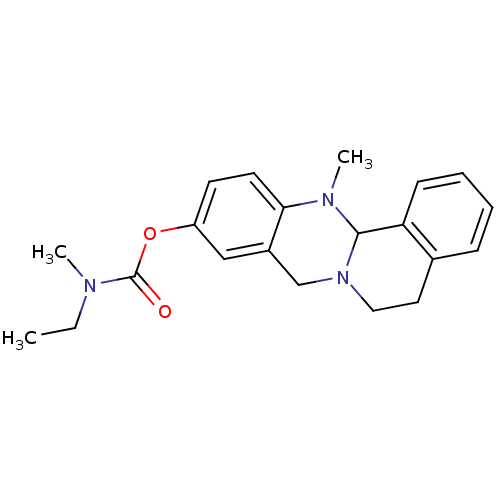 (CHEMBL2152544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50392998 (CHEMBL2152544) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of Electric eel AChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396135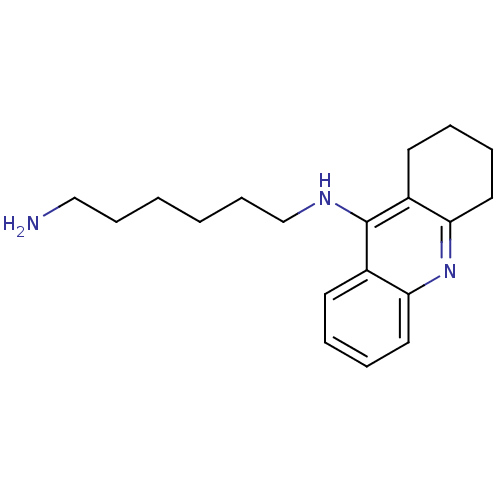 (CHEMBL2171328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396135 (CHEMBL2171328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Eur J Med Chem 81: 15-21 (2014) Article DOI: 10.1016/j.ejmech.2014.05.002 BindingDB Entry DOI: 10.7270/Q24B32VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50160078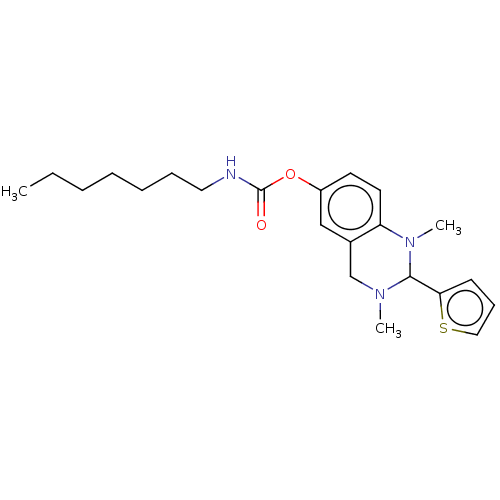 (CHEMBL3787613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured a... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160078 (CHEMBL3787613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160250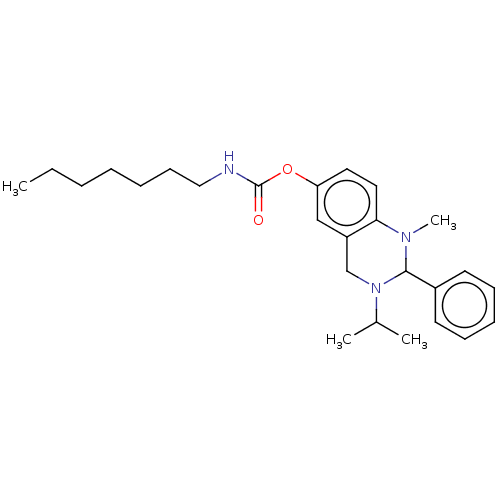 (CHEMBL3785472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160255 (CHEMBL3785189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160077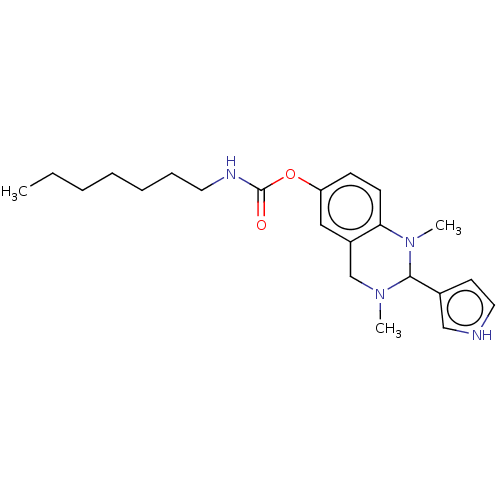 (CHEMBL3785861) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50497922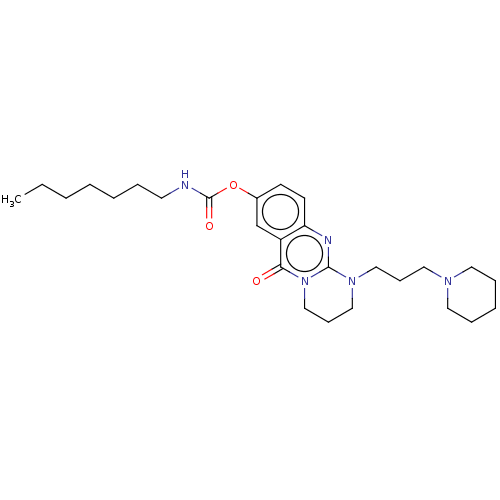 (CHEMBL3323382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50497922 (CHEMBL3323382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160247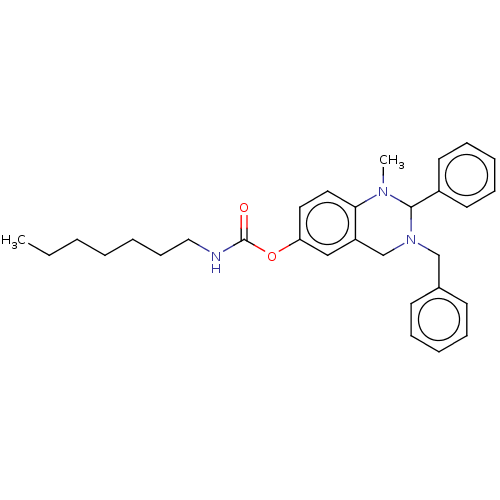 (CHEMBL3786119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396135 (CHEMBL2171328) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396135 (CHEMBL2171328) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160249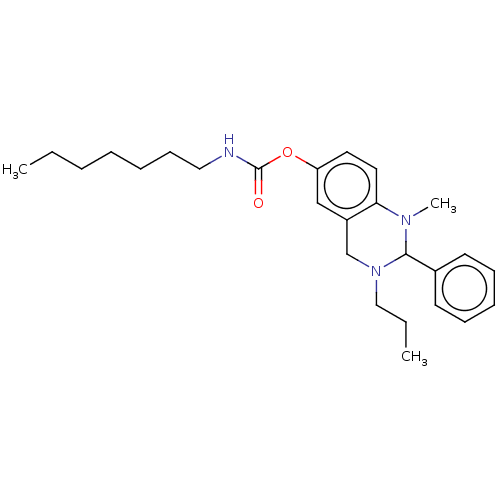 (CHEMBL3785181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160076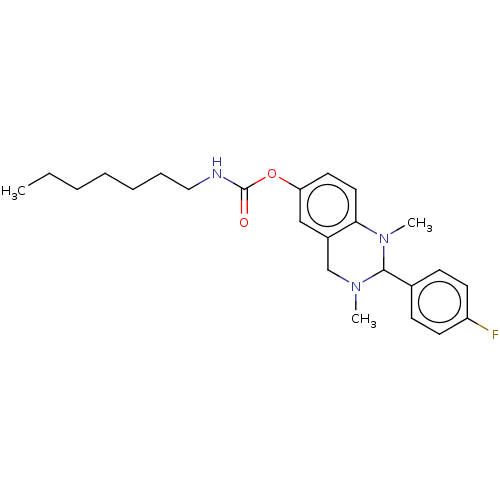 (CHEMBL3787116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393011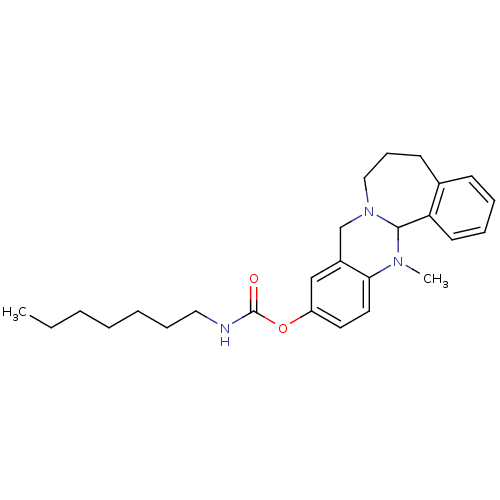 (CHEMBL2152543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393011 (CHEMBL2152543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50401095 (CHEMBL2204447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50401095 (CHEMBL2204447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of horse AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401095 (CHEMBL2204447) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401095 (CHEMBL2204447) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50392999 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 81: 15-21 (2014) Article DOI: 10.1016/j.ejmech.2014.05.002 BindingDB Entry DOI: 10.7270/Q24B32VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50392999 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50016244 (CHEMBL3262489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Eur J Med Chem 81: 15-21 (2014) Article DOI: 10.1016/j.ejmech.2014.05.002 BindingDB Entry DOI: 10.7270/Q24B32VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50497918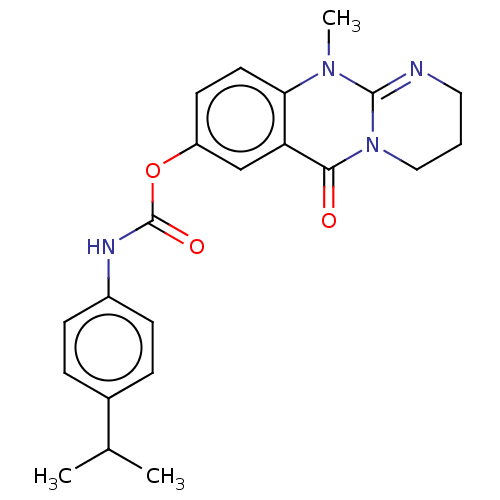 (CHEMBL3323390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160254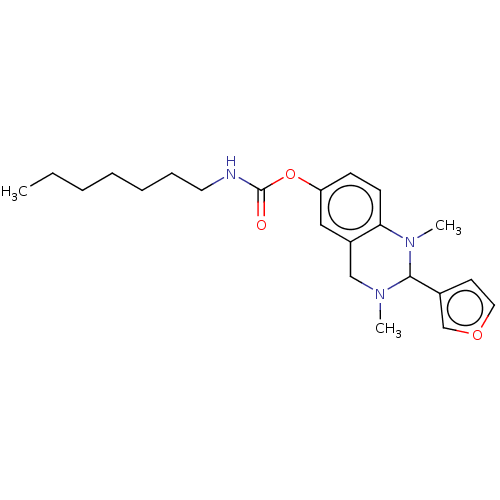 (CHEMBL3786737) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50497918 (CHEMBL3323390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by DTNB based assay | Bioorg Med Chem 22: 5020-34 (2014) Article DOI: 10.1016/j.bmc.2014.06.010 BindingDB Entry DOI: 10.7270/Q2V98C3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160266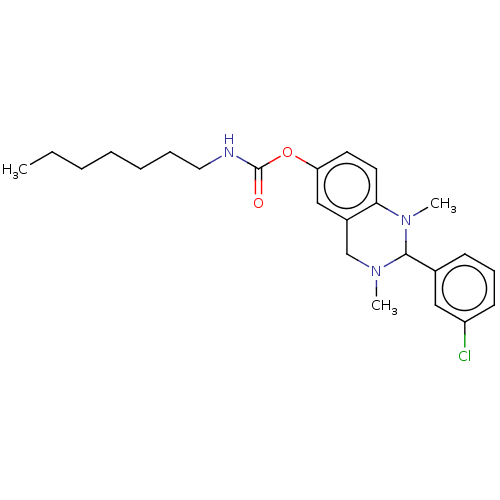 (CHEMBL3787106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401096 (CHEMBL2204448) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401096 (CHEMBL2204448) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by colorimetric Ellman assay | J Med Chem 55: 5231-42 (2012) Article DOI: 10.1021/jm300246n BindingDB Entry DOI: 10.7270/Q2X06868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160268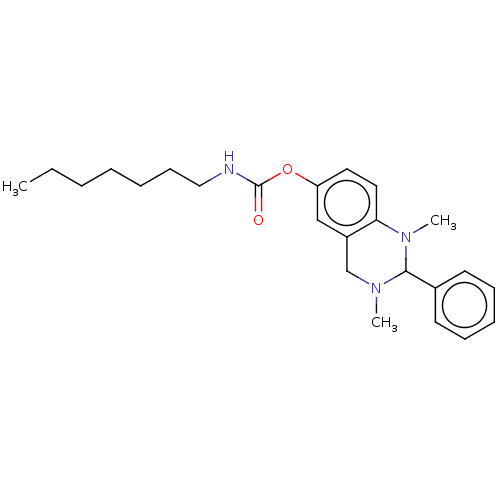 (CHEMBL3786381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50160267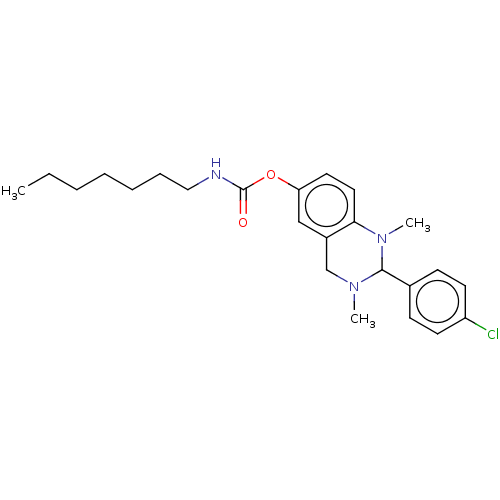 (CHEMBL3785533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393014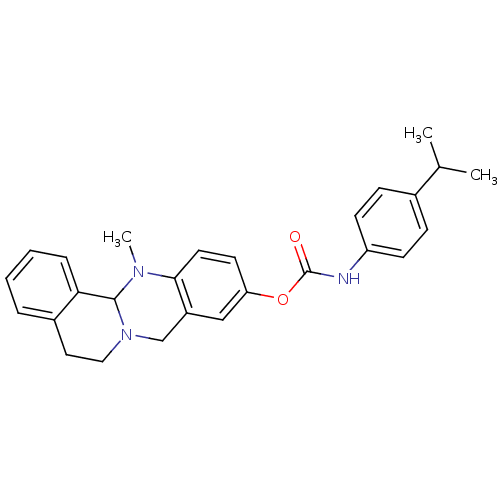 (CHEMBL2152548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393014 (CHEMBL2152548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine as substrate after 2 mins | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 281 total ) | Next | Last >> |