

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM50021359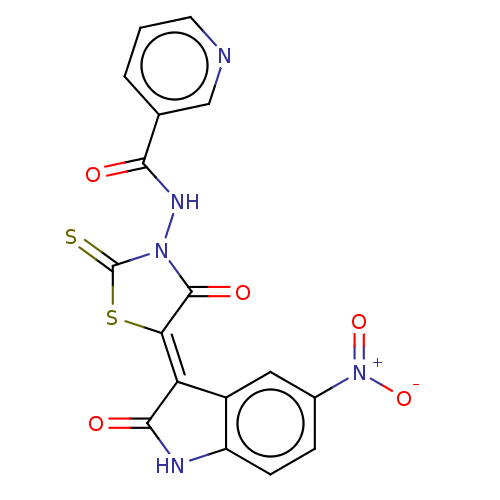 (CHEMBL3288597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli MurD ligase activity using UDP-N-acetylmuramyl-L-alanine as a substrate | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218932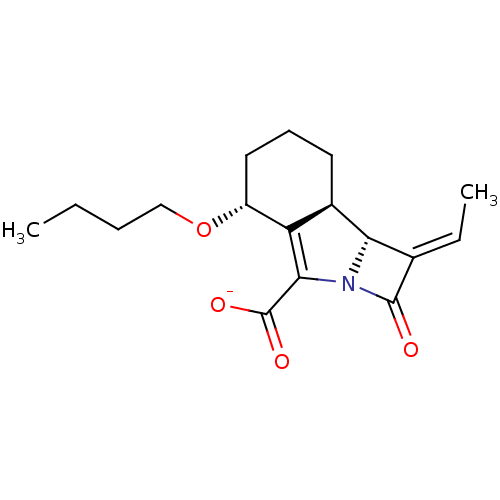 (CHEMBL396998 | sodium (8R,9R)-10(S)-[1(R)-hydroxye...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218936 (CHEMBL397438 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218934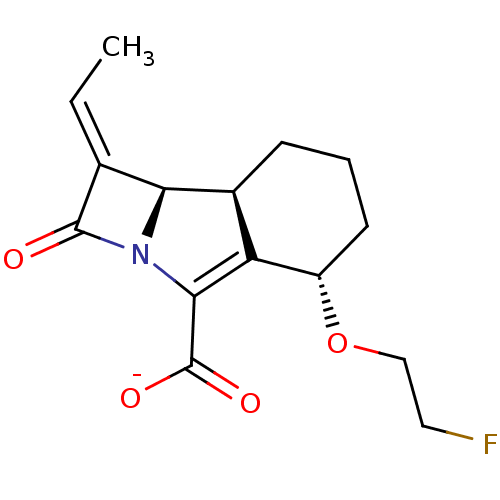 (CHEMBL395017 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218933 (CHEMBL230859 | sodium (8R,9R)-10(E)-ethylidene-4(R...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218939 (CHEMBL230332 | sodium (5S,8aS,8bR)-5-butoxy-1-eth-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218938 (CHEMBL396753 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218935 (CHEMBL396509 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50218937 (CHEMBL230226 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218935 (CHEMBL396509 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218934 (CHEMBL395017 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218935 (CHEMBL396509 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218936 (CHEMBL397438 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218939 (CHEMBL230332 | sodium (5S,8aS,8bR)-5-butoxy-1-eth-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218936 (CHEMBL397438 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218932 (CHEMBL396998 | sodium (8R,9R)-10(S)-[1(R)-hydroxye...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218937 (CHEMBL230226 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218933 (CHEMBL230859 | sodium (8R,9R)-10(E)-ethylidene-4(R...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218933 (CHEMBL230859 | sodium (8R,9R)-10(E)-ethylidene-4(R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218938 (CHEMBL396753 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218938 (CHEMBL396753 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50218937 (CHEMBL230226 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli SHV1 | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218932 (CHEMBL396998 | sodium (8R,9R)-10(S)-[1(R)-hydroxye...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218939 (CHEMBL230332 | sodium (5S,8aS,8bR)-5-butoxy-1-eth-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50218934 (CHEMBL395017 | sodium (8S,9R)-10(E)-ethylidene-4(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d.d. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 50: 4113-21 (2007) Article DOI: 10.1021/jm0703237 BindingDB Entry DOI: 10.7270/Q2FF3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM50021359 (CHEMBL3288597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 43 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26444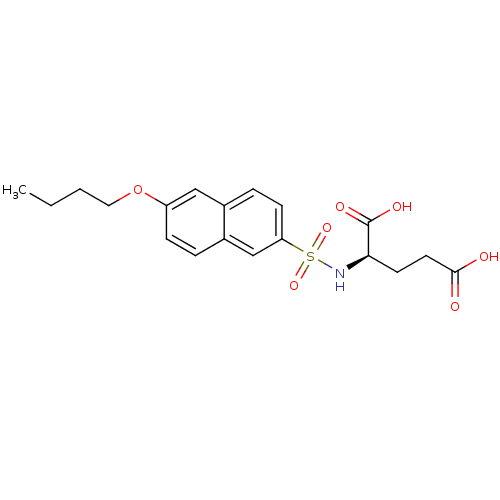 ((2R)-2-[(6-butoxynaphthalene-2-)sulfonamido]pentan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 4 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375218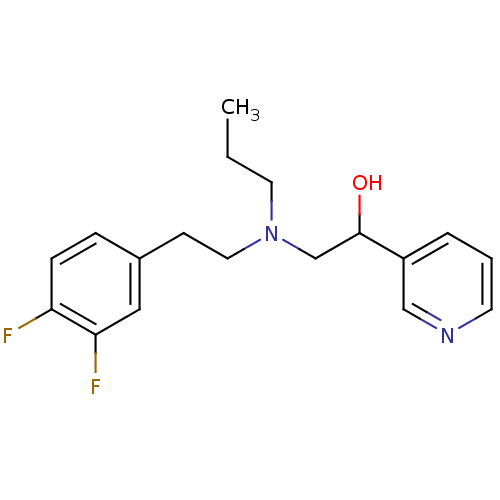 (CHEMBL557376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM50021359 (CHEMBL3288597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375211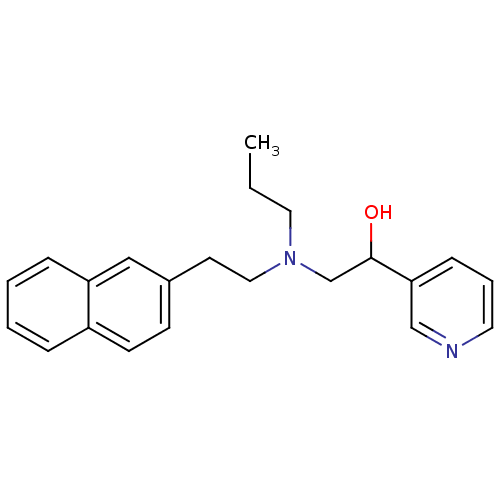 (CHEMBL535872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375212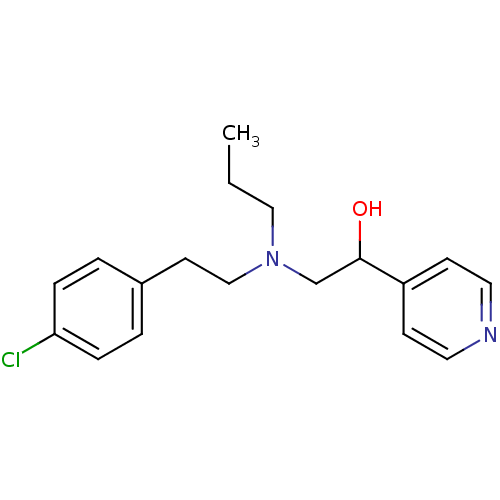 (CHEMBL558189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375213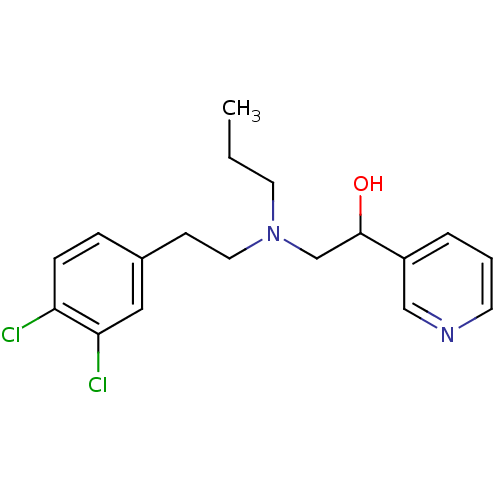 (CHEMBL537002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM25817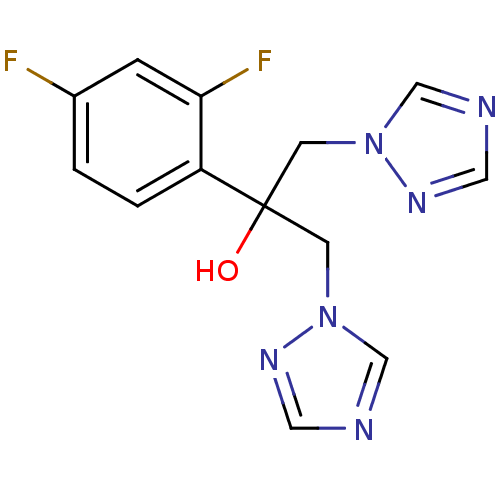 (2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375214 (CHEMBL536772) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375215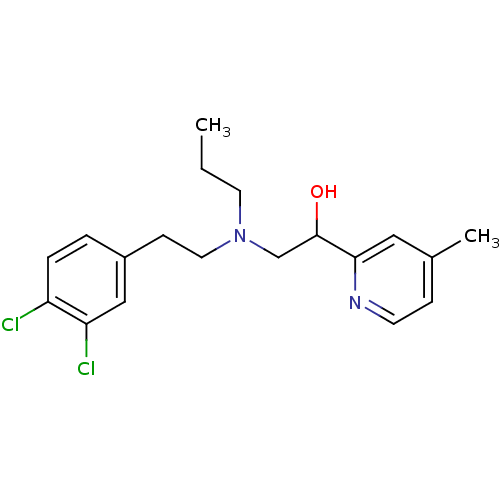 (CHEMBL538591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375216 (CHEMBL537231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol 14-alpha demethylase (Homo sapiens (Human)) | BDBM50375217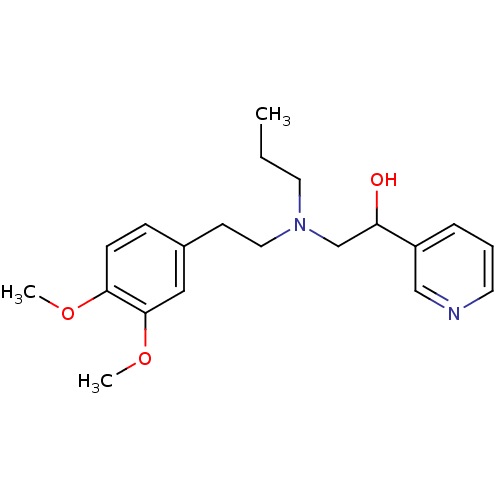 (CHEMBL537003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a |
Lek Pharmaceuticals d. d. Curated by ChEMBL | Assay Description Binding affinity to human His-tagged CYP51 expressed in Escherichia coli | Bioorg Med Chem 16: 209-21 (2008) Article DOI: 10.1016/j.bmc.2007.10.001 BindingDB Entry DOI: 10.7270/Q2TQ62DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM50021359 (CHEMBL3288597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 71 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26444 ((2R)-2-[(6-butoxynaphthalene-2-)sulfonamido]pentan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 123 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26455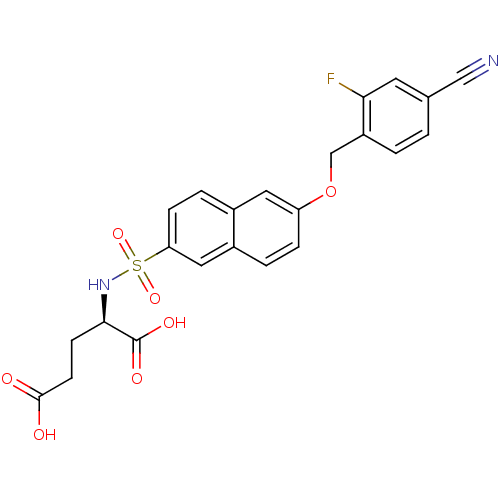 ((2R)-2-({6-[(4-cyano-2-fluorophenyl)methoxy]naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 9 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26455 ((2R)-2-({6-[(4-cyano-2-fluorophenyl)methoxy]naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 13 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26455 ((2R)-2-({6-[(4-cyano-2-fluorophenyl)methoxy]naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 56 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM50021359 (CHEMBL3288597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a |
Slovenia National Institute of Chemistry Curated by ChEMBL | Assay Description Binding affinity to 6x His-tagged and 15N/13C-labeled Escherichia coli MurD ligase measured at NMR signal 4 by 1H/13C-HSQC 2D NMR spectroscopy | Eur J Med Chem 83: 92-101 (2014) Article DOI: 10.1016/j.ejmech.2014.06.021 BindingDB Entry DOI: 10.7270/Q25D8TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||