

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50190362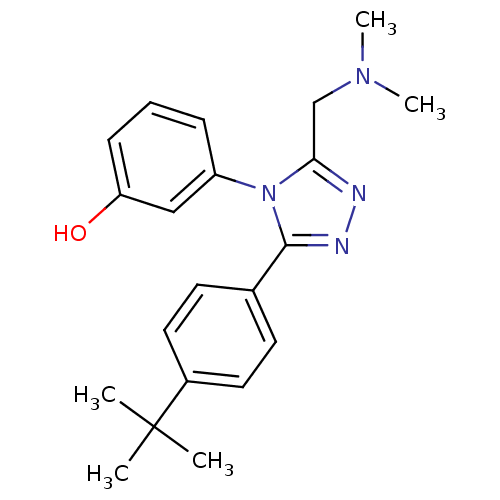 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50190374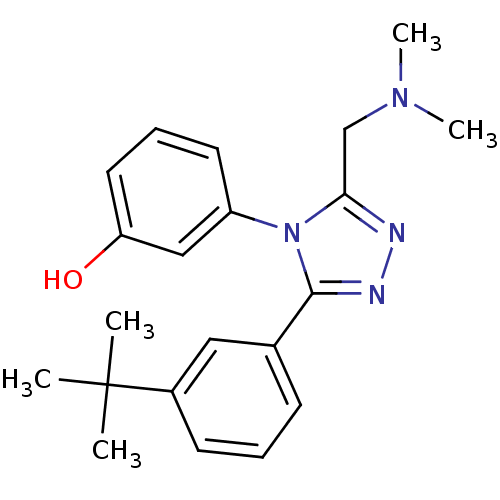 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50190374 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50190362 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190362 (1-{3-[4-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50190374 (1-{3-[3-(tert-butyl) phenyl]-5-N,N-dimethylaminome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304075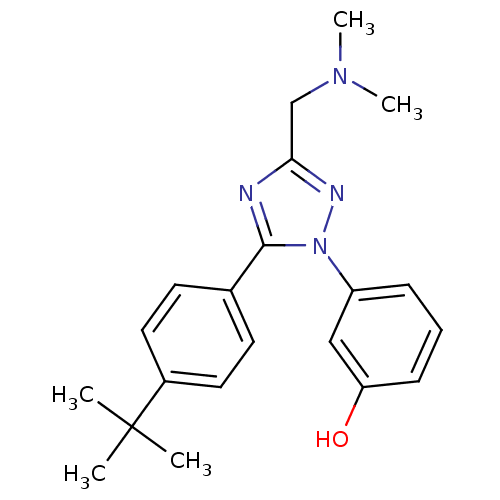 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304084 (3-(2-(3-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304084 (3-(2-(3-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304082 (3-(5-(3-tert-Butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304076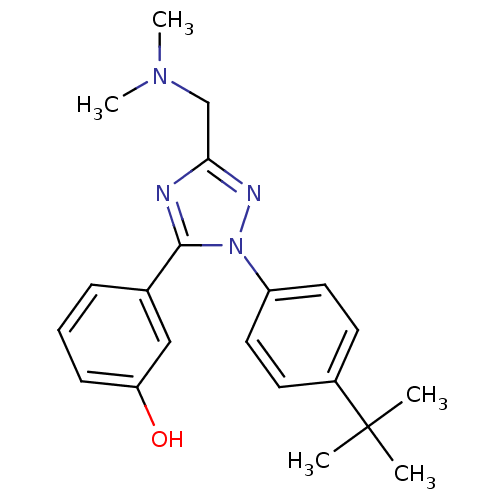 (3-(2-(4-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304084 (3-(2-(3-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304083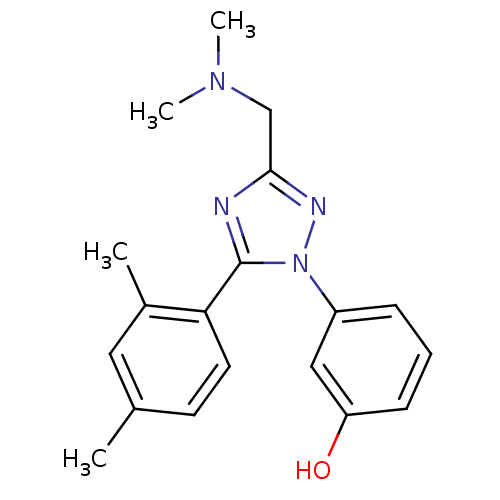 (3-(3-((Dimethylamino)methyl)-5-(2,4-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM218671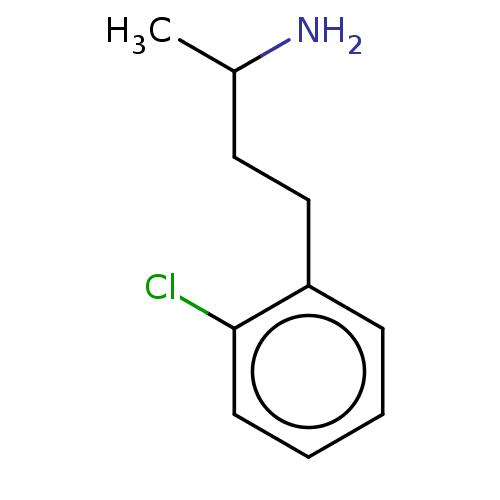 (US9289400, ES609) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University; Rutgers, The State University of New Jersey US Patent | Assay Description Functional efficacy was determined by assessing the ability of PBZI and ES609 to inhibit monoamine transporter uptake activity. | US Patent US9289400 (2016) BindingDB Entry DOI: 10.7270/Q29S1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304082 (3-(5-(3-tert-Butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304083 (3-(3-((Dimethylamino)methyl)-5-(2,4-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304076 (3-(2-(4-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM218672 (US9289400, PBZI) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University; Rutgers, The State University of New Jersey US Patent | Assay Description Functional efficacy was determined by assessing the ability of PBZI and ES609 to inhibit monoamine transporter uptake activity. | US Patent US9289400 (2016) BindingDB Entry DOI: 10.7270/Q29S1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304078 ((1-(3-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM218672 (US9289400, PBZI) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University; Rutgers, The State University of New Jersey US Patent | Assay Description Functional efficacy was determined by assessing the ability of PBZI and ES609 to inhibit monoamine transporter uptake activity. | US Patent US9289400 (2016) BindingDB Entry DOI: 10.7270/Q29S1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT7 receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304081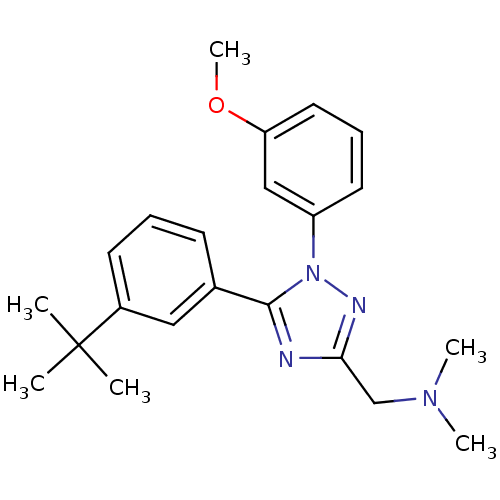 ((5-(3-tert-Butylphenyl)-1-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304080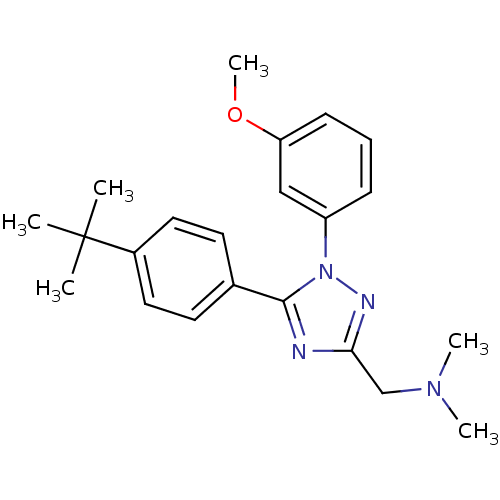 ((5-(4-tert-Butylphenyl)-1-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304083 (3-(3-((Dimethylamino)methyl)-5-(2,4-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304082 (3-(5-(3-tert-Butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT2B receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM218671 (US9289400, ES609) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University; Rutgers, The State University of New Jersey US Patent | Assay Description Functional efficacy was determined by assessing the ability of PBZI and ES609 to inhibit monoamine transporter uptake activity. | US Patent US9289400 (2016) BindingDB Entry DOI: 10.7270/Q29S1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of muscarinic M3 receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of muscarinic M4 receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of muscarinic M5 receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of DAT | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of NET | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of beta2 adrenergic receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of alpha2B adrenergic receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT1E receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT1D receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT1B receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50304075 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT1A receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304076 (3-(2-(4-tert-Butylphenyl)-5-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304077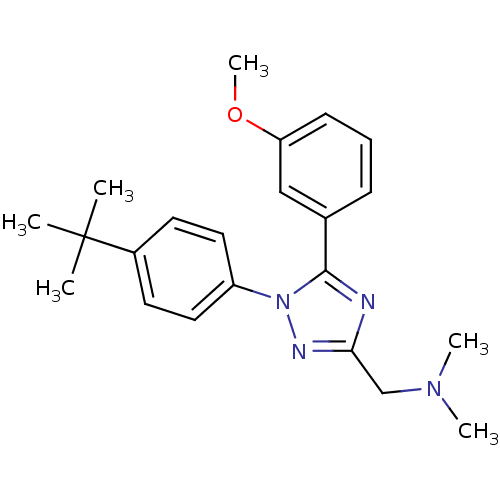 ((1-(4-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304077 ((1-(4-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304077 ((1-(4-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse delta opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304078 ((1-(3-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50304078 ((1-(3-tert-Butylphenyl)-5-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from mouse mu opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304079 ((5-(2,4-Dimethylphenyl)-1-(3-methoxyphenyl)-1H-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from rat kappa opioid receptor expressed in HEK293 cells by liquid scintillation counting | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |