

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455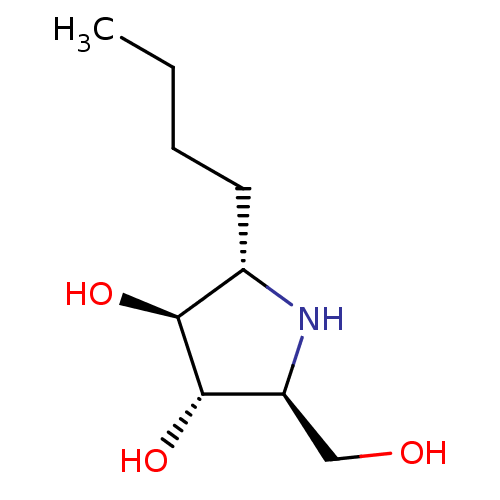 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50242271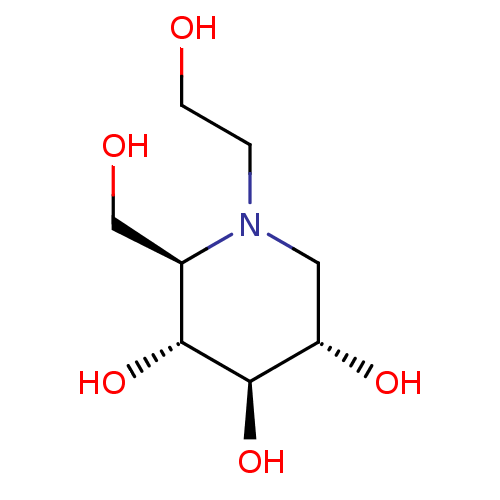 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50049730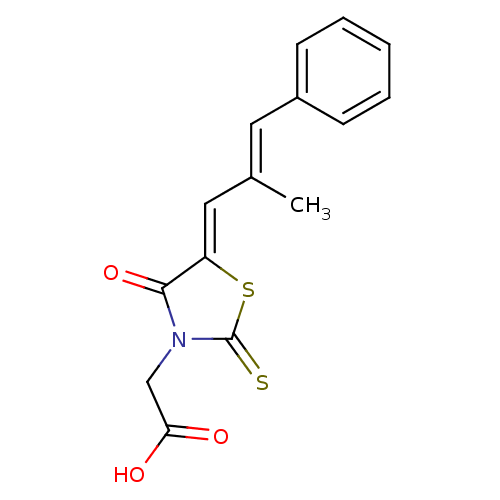 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457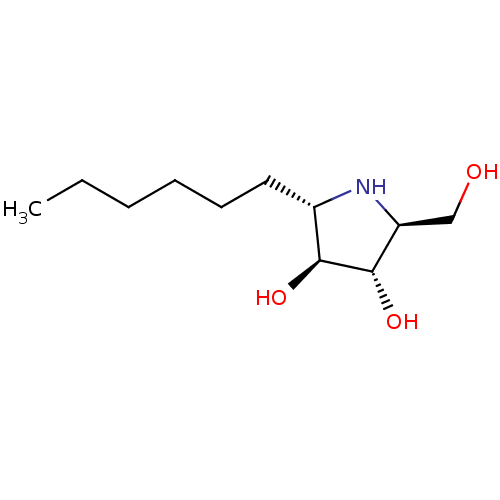 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044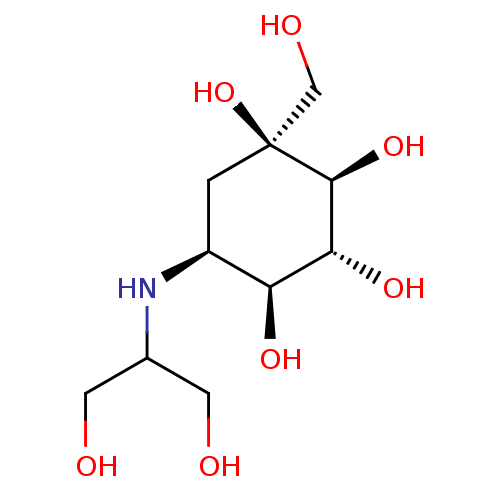 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18363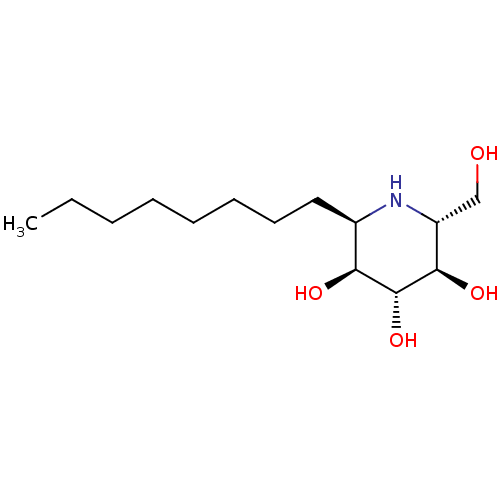 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363064 (CHEMBL1944855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363053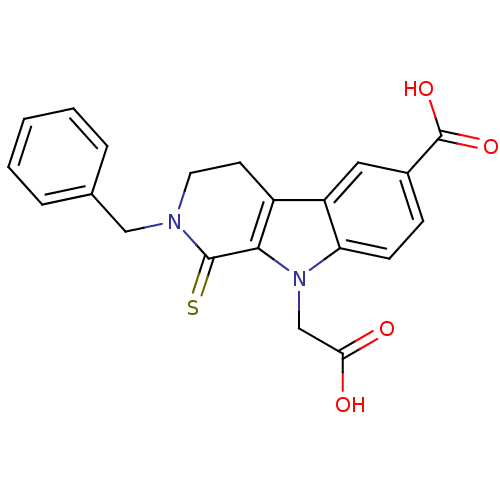 (CHEMBL1944862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465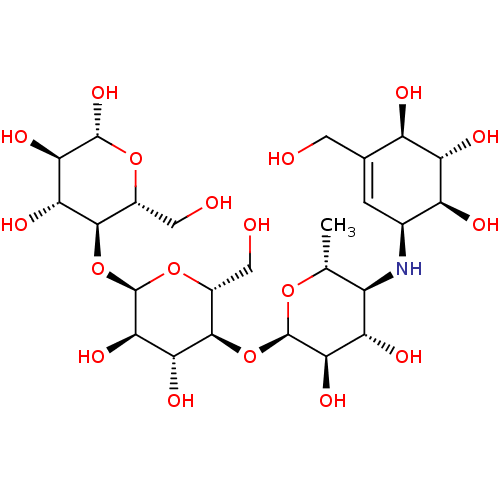 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399654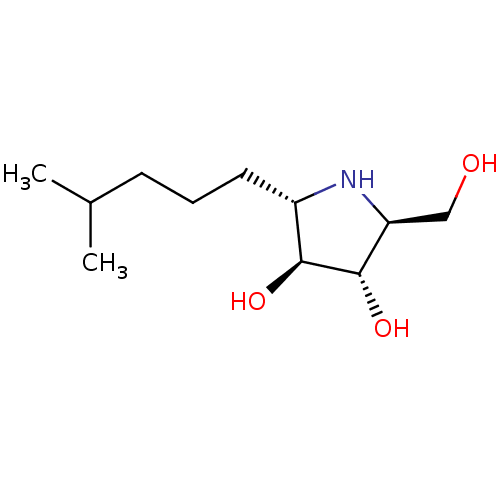 (CHEMBL2177691) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363063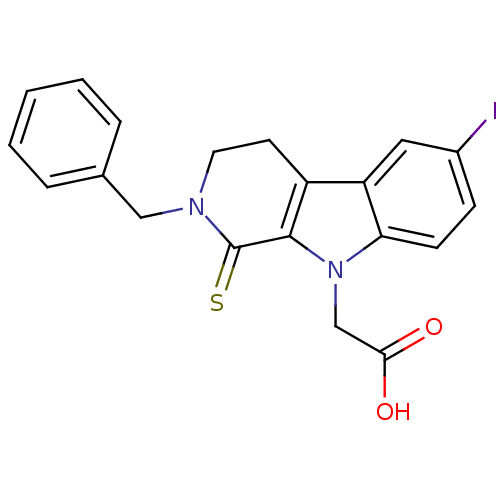 (CHEMBL1944854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333456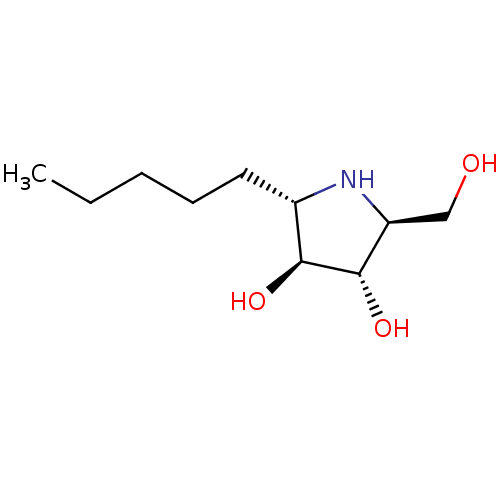 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-pentylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363066 (CHEMBL1944857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363070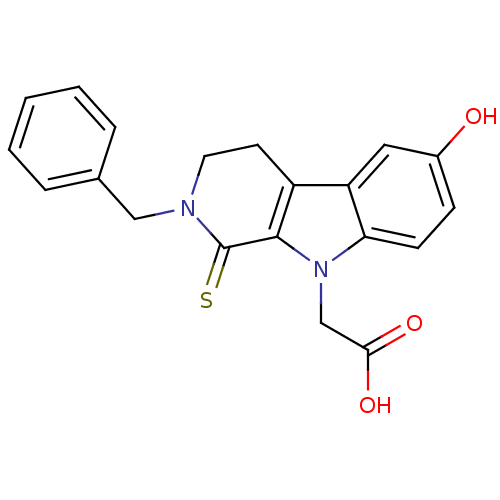 (CHEMBL1944861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363065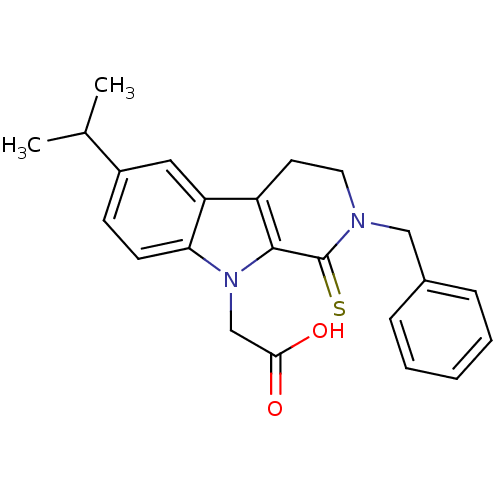 (CHEMBL1944856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399655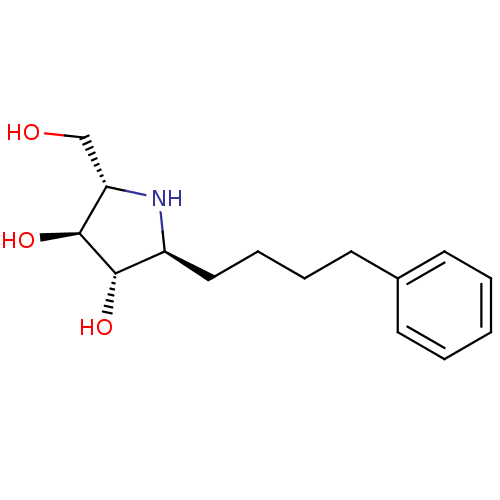 (CHEMBL2177690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363054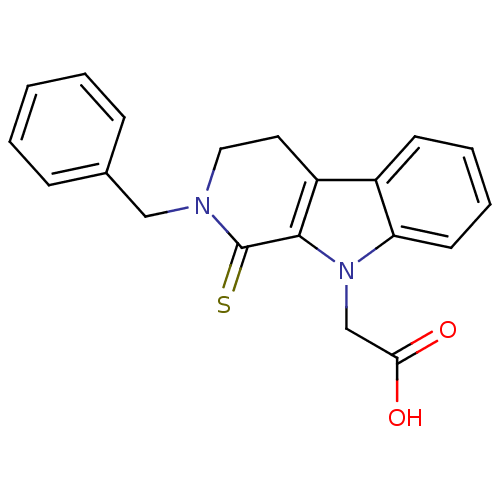 (CHEMBL1946959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363055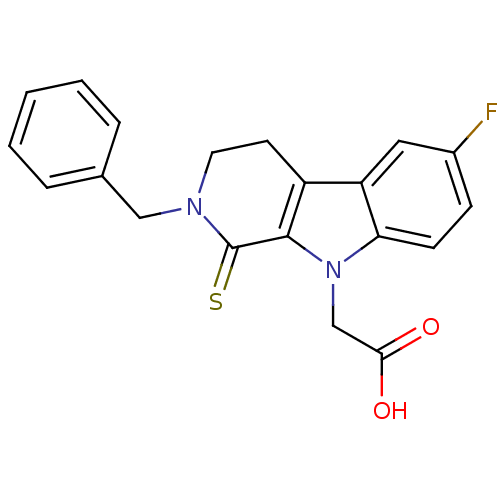 (CHEMBL1946960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363061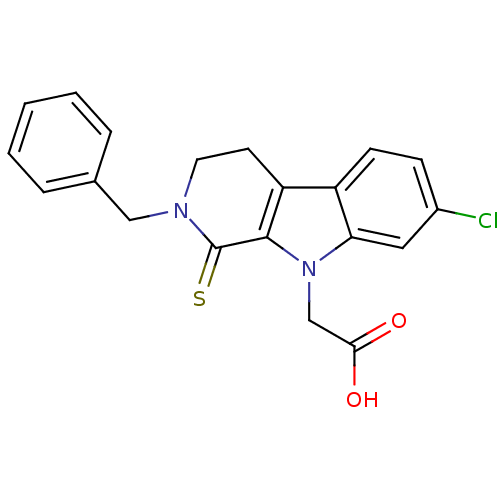 (CHEMBL1946966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399654 (CHEMBL2177691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333458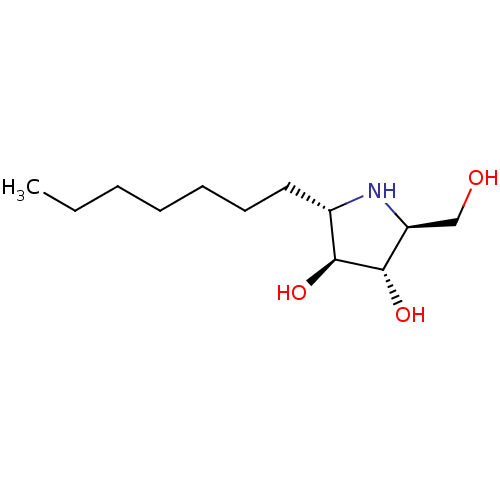 ((2S,3S,4S,5S)-2-heptyl-5-(hydroxymethyl)pyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333454 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-propylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50363057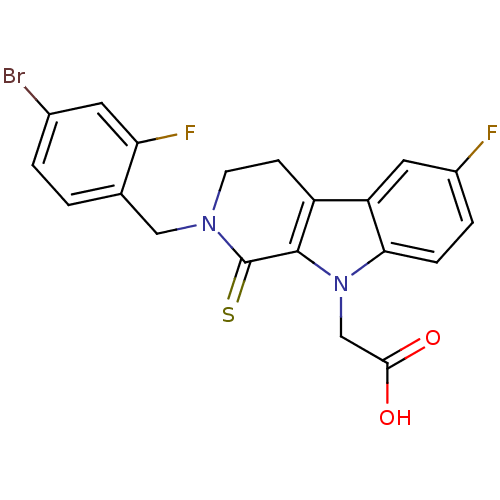 (CHEMBL1946962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant N-His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18362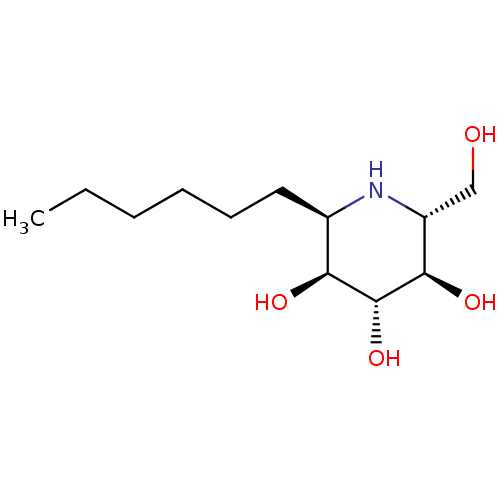 ((2R,3S,4R,5R,6R)-2-hexyl-6-(hydroxymethyl)piperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399655 (CHEMBL2177690) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333459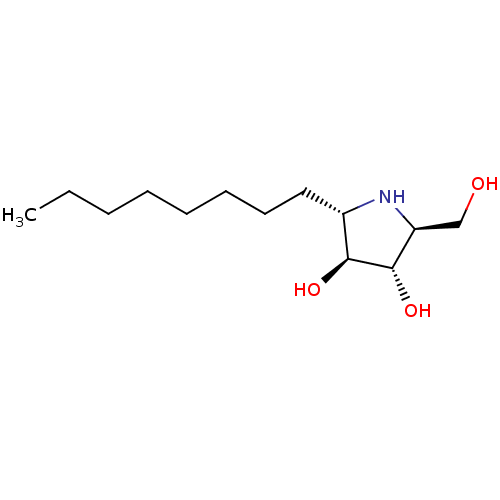 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-octylpyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363060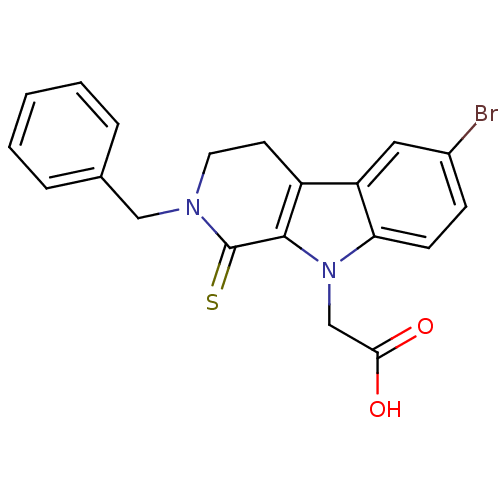 (CHEMBL1946965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50049730 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant N-His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50363062 (CHEMBL1944853) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1B1 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50375511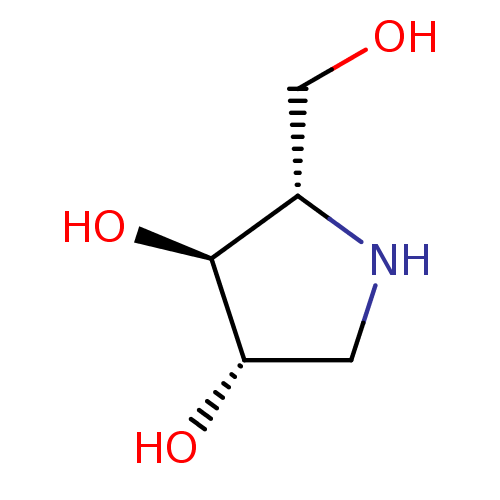 (CHEMBL406973) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50263044 (CHEMBL476960 | Voglibose) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333458 ((2S,3S,4S,5S)-2-heptyl-5-(hydroxymethyl)pyrrolidin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50363069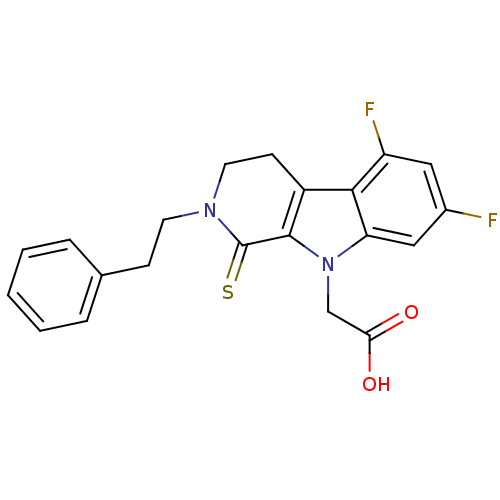 (CHEMBL1944860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant N-His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333459 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-octylpyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18361 ((2R,3S,4R,5R,6R)-2-butyl-6-(hydroxymethyl)piperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50363067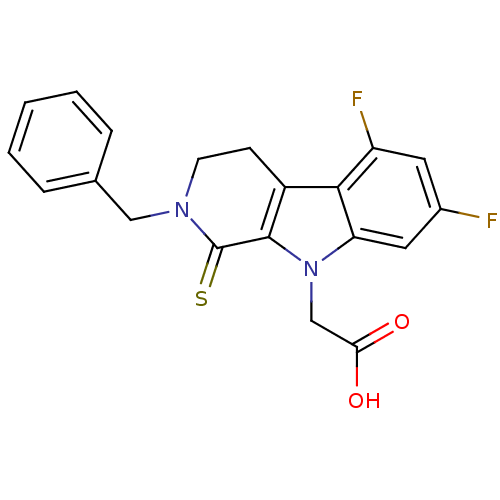 (CHEMBL1944858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant N-His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333457 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50375510 (CHEMBL405957) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399650 (CHEMBL2177689) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399653 (CHEMBL2177692) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333453 ((2S,3S,4S,5S)-2-ethyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50363072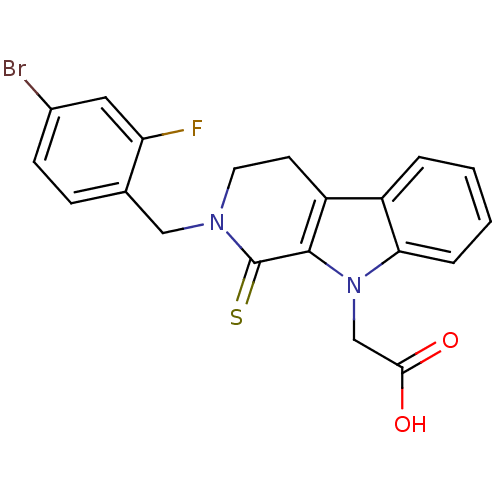 (CHEMBL1944852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant N-His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using pyridine-3-aldehyde as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333456 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-pentylpyrrolidin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50375510 (CHEMBL405957) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333461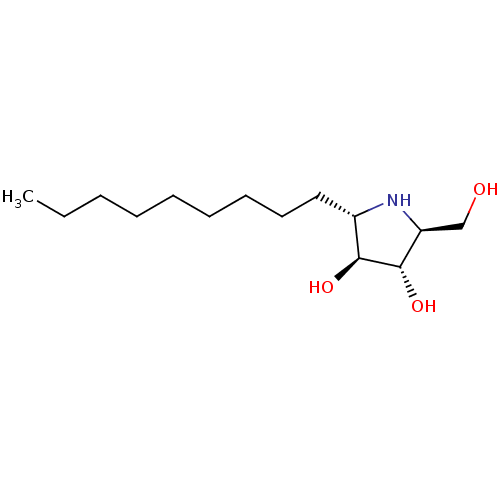 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-nonylpyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |