 Found 44 hits with Last Name = 'kouji' and Initial = 'h'
Found 44 hits with Last Name = 'kouji' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151161
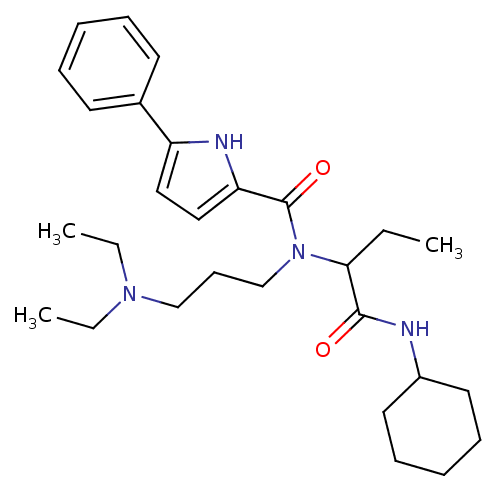
(5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...)Show SMILES CCC(N(CCCN(CC)CC)C(=O)c1ccc([nH]1)-c1ccccc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C28H42N4O2/c1-4-26(27(33)29-23-16-11-8-12-17-23)32(21-13-20-31(5-2)6-3)28(34)25-19-18-24(30-25)22-14-9-7-10-15-22/h7,9-10,14-15,18-19,23,26,30H,4-6,8,11-13,16-17,20-21H2,1-3H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186367
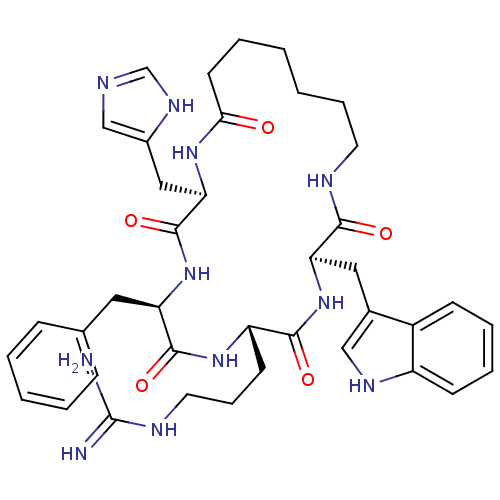
(CHEMBL379959 | c(his-D-phe-arg-trp-Ahp))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C39H51N11O5/c40-39(41)44-18-10-15-30-36(53)50-32(20-26-22-45-29-14-8-7-13-28(26)29)35(52)43-17-9-2-1-6-16-34(51)47-33(21-27-23-42-24-46-27)38(55)49-31(37(54)48-30)19-25-11-4-3-5-12-25/h3-5,7-8,11-14,22-24,30-33,45H,1-2,6,9-10,15-21H2,(H,42,46)(H,43,52)(H,47,51)(H,48,54)(H,49,55)(H,50,53)(H4,40,41,44)/t30-,31+,32+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human cloned MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151159
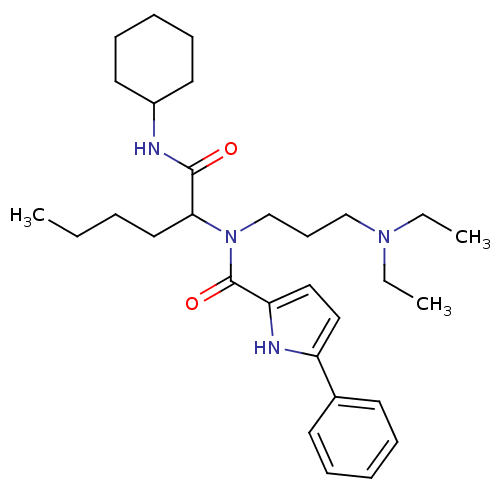
(5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...)Show SMILES CCCCC(N(CCCN(CC)CC)C(=O)c1ccc([nH]1)-c1ccccc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C30H46N4O2/c1-4-7-19-28(29(35)31-25-17-12-9-13-18-25)34(23-14-22-33(5-2)6-3)30(36)27-21-20-26(32-27)24-15-10-8-11-16-24/h8,10-11,15-16,20-21,25,28,32H,4-7,9,12-14,17-19,22-23H2,1-3H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186371
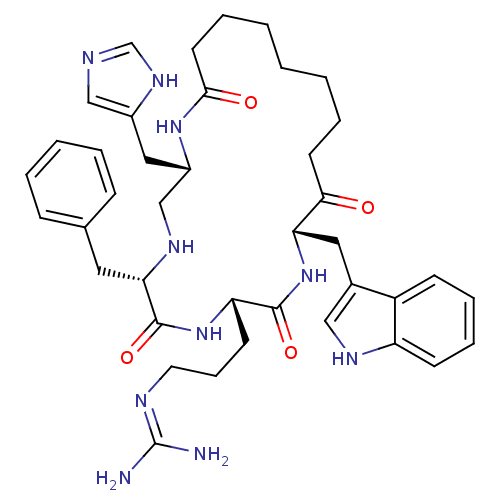
(CHEMBL264120 | c(his-D-phe-arg-trp-Aoc))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC[C@H](Cc2cnc[nH]2)NC(=O)CCCCCCCC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:21.22,40.42,7.6,wD:11.11,(14.11,-13.67,;13.11,-12.52,;13.62,-11.08,;11.62,-12.8,;10.62,-11.65,;9.13,-11.93,;8.13,-10.78,;6.64,-11.06,;5.6,-9.87,;4.18,-9.13,;4.11,-7.53,;2.85,-9.99,;2.84,-8.38,;1.44,-7.58,;1.43,-5.97,;.03,-5.18,;-1.36,-5.99,;-1.33,-7.78,;.06,-8.4,;1.47,-10.67,;.04,-10.09,;-1.33,-9.3,;-2.1,-7.97,;-3.62,-7.97,;-4.53,-6.74,;-5.97,-7.21,;-5.97,-8.73,;-4.52,-9.2,;-2.59,-10.19,;-2.67,-11.73,;-4.47,-12.71,;-1.28,-12.48,;-1.28,-14.08,;.11,-14.88,;1.49,-14.07,;2.87,-14.87,;2.87,-16.48,;4.47,-17.01,;5.02,-15.59,;4.11,-14.39,;6.58,-15.28,;7.56,-16.44,;7.05,-17.88,;5.59,-18.3,;5.55,-19.83,;6.98,-20.34,;7.55,-21.74,;9.06,-21.95,;9.99,-20.74,;9.41,-19.34,;7.91,-19.14,;7.02,-13.81,;6.1,-12.57,;4.53,-12.87,)| Show InChI InChI=1S/C40H54N10O4/c41-40(42)44-19-11-16-33-38(53)50-34(21-28-23-45-32-15-10-9-14-31(28)32)36(51)17-7-2-1-3-8-18-37(52)48-30(22-29-24-43-26-47-29)25-46-35(39(54)49-33)20-27-12-5-4-6-13-27/h4-6,9-10,12-15,23-24,26,30,33-35,45-46H,1-3,7-8,11,16-22,25H2,(H,43,47)(H,48,52)(H,49,54)(H,50,53)(H4,41,42,44)/t30-,33-,34-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human cloned MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186370
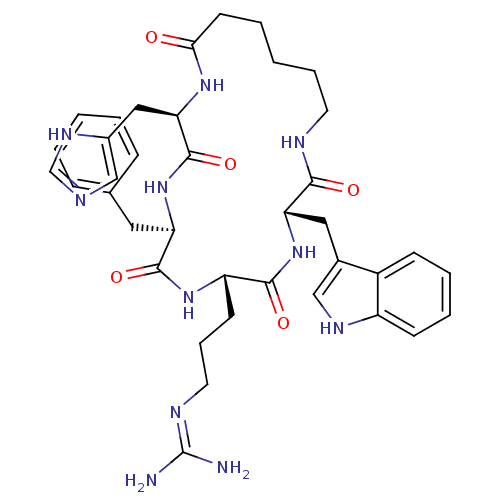
(CHEMBL379627 | c(his-D-phe-arg-trp-Ahx))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:40.42,7.6,(.29,-13.95,;1.12,-12.66,;.41,-11.3,;2.65,-12.73,;3.47,-11.44,;2.77,-10.08,;3.6,-8.8,;2.89,-7.42,;1.34,-6.07,;-.11,-7.87,;-.39,-9.37,;-1.55,-7.32,;-2.32,-8.66,;-3.85,-8.66,;-4.63,-10,;-6.16,-10,;-6.92,-8.68,;-6.16,-7.36,;-4.63,-7.36,;-1.93,-5.49,;-3.54,-5.03,;-4.98,-5.63,;-3.9,-3.54,;-5.44,-3.64,;-6.35,-2.39,;-7.88,-2.39,;-8.36,-.92,;-7.11,-.03,;-5.87,-.92,;-2.4,-2.19,;-3.11,-.61,;-4.37,.26,;-2.02,.48,;-.16,-.65,;.87,1.42,;1.85,-.18,;3.71,.36,;3.41,-1.72,;5.32,-2.22,;6.82,-1.87,;5.42,-3.75,;6.94,-3.91,;7.57,-5.32,;6.8,-6.65,;7.83,-7.8,;9.23,-7.17,;10.64,-7.8,;11.89,-6.89,;11.72,-5.35,;10.32,-4.73,;9.08,-5.63,;3.46,-4.56,;4.14,-6.49,;5.23,-7.58,)| Show InChI InChI=1S/C38H49N11O5/c39-38(40)43-17-9-14-29-35(52)49-31(19-25-21-44-28-13-7-6-12-27(25)28)34(51)42-16-8-2-5-15-33(50)46-32(20-26-22-41-23-45-26)37(54)48-30(36(53)47-29)18-24-10-3-1-4-11-24/h1,3-4,6-7,10-13,21-23,29-32,44H,2,5,8-9,14-20H2,(H,41,45)(H,42,51)(H,46,50)(H,47,53)(H,48,54)(H,49,52)(H4,39,40,43)/t29-,30+,31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human cloned MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151163
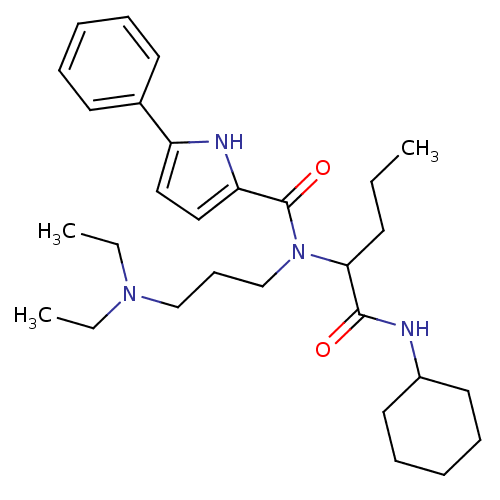
(5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...)Show SMILES CCCC(N(CCCN(CC)CC)C(=O)c1ccc([nH]1)-c1ccccc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H44N4O2/c1-4-14-27(28(34)30-24-17-11-8-12-18-24)33(22-13-21-32(5-2)6-3)29(35)26-20-19-25(31-26)23-15-9-7-10-16-23/h7,9-10,15-16,19-20,24,27,31H,4-6,8,11-14,17-18,21-22H2,1-3H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151168
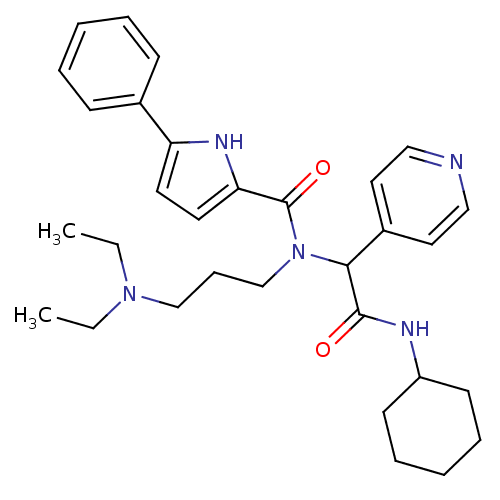
(5-Phenyl-1H-pyrrole-2-carboxylic acid (cyclohexylc...)Show SMILES CCN(CC)CCCN(C(C(=O)NC1CCCCC1)c1ccncc1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C31H41N5O2/c1-3-35(4-2)22-11-23-36(31(38)28-17-16-27(34-28)24-12-7-5-8-13-24)29(25-18-20-32-21-19-25)30(37)33-26-14-9-6-10-15-26/h5,7-8,12-13,16-21,26,29,34H,3-4,6,9-11,14-15,22-23H2,1-2H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151160
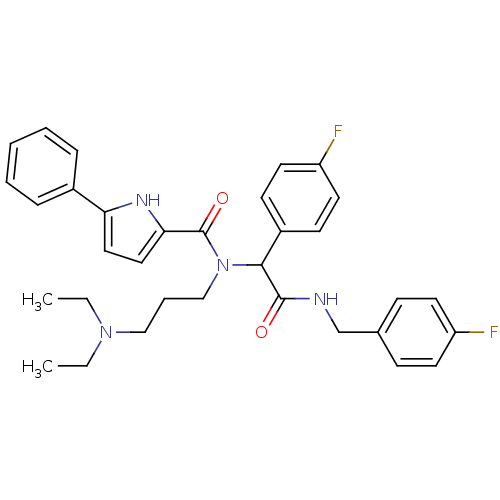
(5-Phenyl-1H-pyrrole-2-carboxylic acid (3-diethylam...)Show SMILES CCN(CC)CCCN(C(C(=O)NCc1ccc(F)cc1)c1ccc(F)cc1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C33H36F2N4O2/c1-3-38(4-2)21-8-22-39(33(41)30-20-19-29(37-30)25-9-6-5-7-10-25)31(26-13-17-28(35)18-14-26)32(40)36-23-24-11-15-27(34)16-12-24/h5-7,9-20,31,37H,3-4,8,21-23H2,1-2H3,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151169
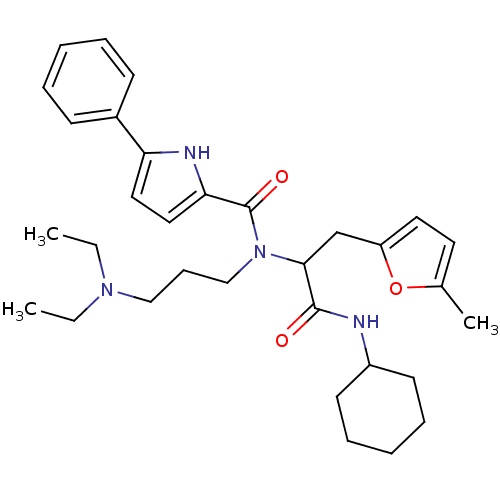
(5-Phenyl-1H-pyrrole-2-carboxylic acid [1-cyclohexy...)Show SMILES CCN(CC)CCCN(C(Cc1ccc(C)o1)C(=O)NC1CCCCC1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C32H44N4O3/c1-4-35(5-2)21-12-22-36(32(38)29-20-19-28(34-29)25-13-8-6-9-14-25)30(23-27-18-17-24(3)39-27)31(37)33-26-15-10-7-11-16-26/h6,8-9,13-14,17-20,26,30,34H,4-5,7,10-12,15-16,21-23H2,1-3H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151158
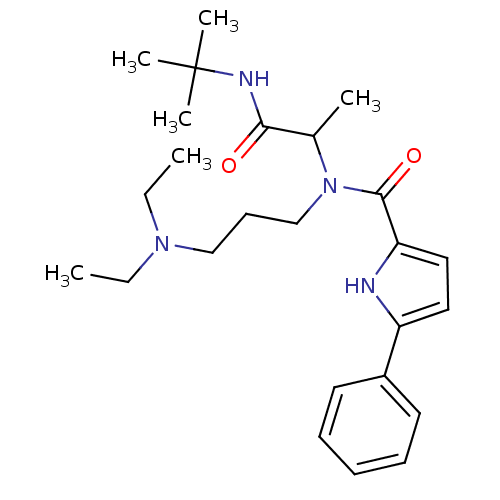
(5-Phenyl-1H-pyrrole-2-carboxylic acid (1-tert-buty...)Show SMILES CCN(CC)CCCN(C(C)C(=O)NC(C)(C)C)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C25H38N4O2/c1-7-28(8-2)17-12-18-29(19(3)23(30)27-25(4,5)6)24(31)22-16-15-21(26-22)20-13-10-9-11-14-20/h9-11,13-16,19,26H,7-8,12,17-18H2,1-6H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151167
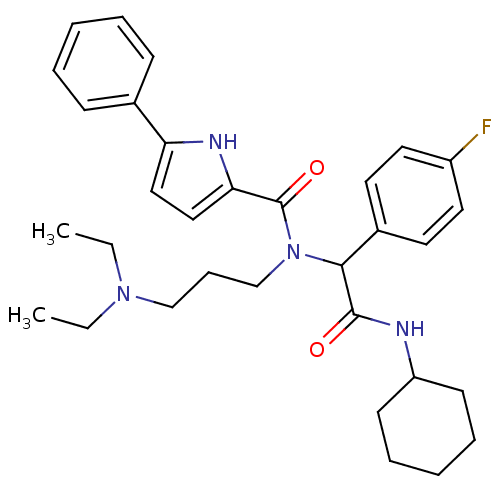
(5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...)Show SMILES CCN(CC)CCCN(C(C(=O)NC1CCCCC1)c1ccc(F)cc1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C32H41FN4O2/c1-3-36(4-2)22-11-23-37(32(39)29-21-20-28(35-29)24-12-7-5-8-13-24)30(25-16-18-26(33)19-17-25)31(38)34-27-14-9-6-10-15-27/h5,7-8,12-13,16-21,27,30,35H,3-4,6,9-11,14-15,22-23H2,1-2H3,(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151164
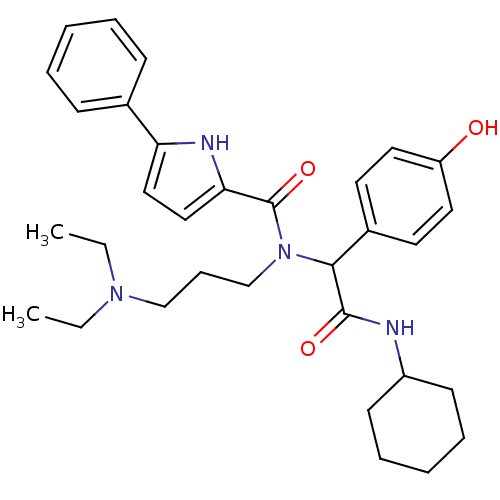
(5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...)Show SMILES CCN(CC)CCCN(C(C(=O)NC1CCCCC1)c1ccc(O)cc1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C32H42N4O3/c1-3-35(4-2)22-11-23-36(32(39)29-21-20-28(34-29)24-12-7-5-8-13-24)30(25-16-18-27(37)19-17-25)31(38)33-26-14-9-6-10-15-26/h5,7-8,12-13,16-21,26,30,34,37H,3-4,6,9-11,14-15,22-23H2,1-2H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151157
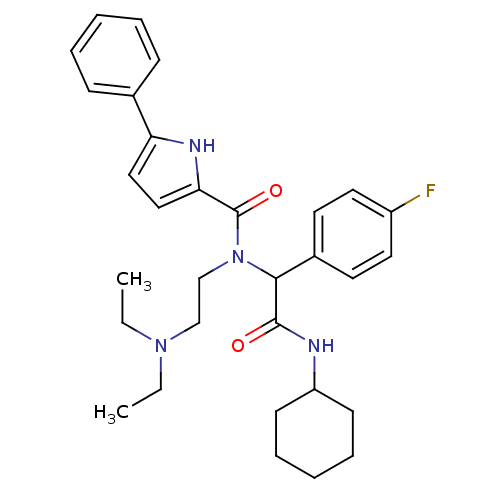
(5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...)Show SMILES CCN(CC)CCN(C(C(=O)NC1CCCCC1)c1ccc(F)cc1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C31H39FN4O2/c1-3-35(4-2)21-22-36(31(38)28-20-19-27(34-28)23-11-7-5-8-12-23)29(24-15-17-25(32)18-16-24)30(37)33-26-13-9-6-10-14-26/h5,7-8,11-12,15-20,26,29,34H,3-4,6,9-10,13-14,21-22H2,1-2H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151165
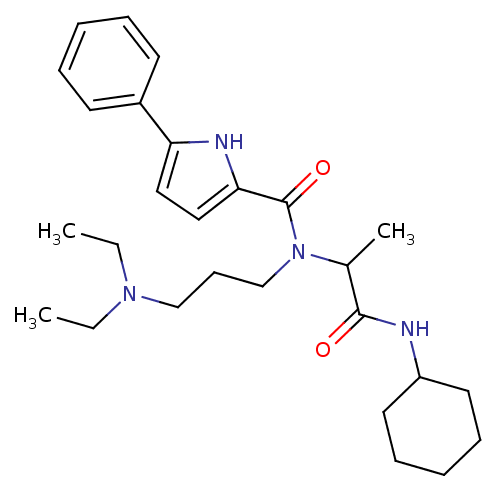
(5-Phenyl-1H-pyrrole-2-carboxylic acid (1-cyclohexy...)Show SMILES CCN(CC)CCCN(C(C)C(=O)NC1CCCCC1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C27H40N4O2/c1-4-30(5-2)19-12-20-31(21(3)26(32)28-23-15-10-7-11-16-23)27(33)25-18-17-24(29-25)22-13-8-6-9-14-22/h6,8-9,13-14,17-18,21,23,29H,4-5,7,10-12,15-16,19-20H2,1-3H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151170

(CHEMBL185887 | N-[Cyclohexylcarbamoyl-(4-fluoro-ph...)Show SMILES CCN(CC)CCCN(C(C(=O)NC1CCCCC1)c1ccc(F)cc1)C(=O)CCN1CCCc2ccccc12 Show InChI InChI=1S/C33H47FN4O2/c1-3-36(4-2)22-11-24-38(31(39)21-25-37-23-10-13-26-12-8-9-16-30(26)37)32(27-17-19-28(34)20-18-27)33(40)35-29-14-6-5-7-15-29/h8-9,12,16-20,29,32H,3-7,10-11,13-15,21-25H2,1-2H3,(H,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151166
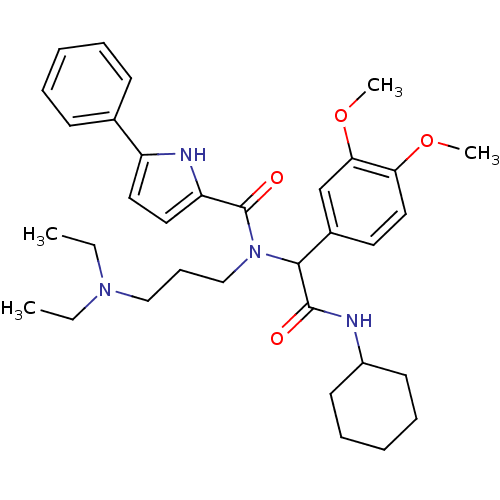
(5-Phenyl-1H-pyrrole-2-carboxylic acid [cyclohexylc...)Show SMILES CCN(CC)CCCN(C(C(=O)NC1CCCCC1)c1ccc(OC)c(OC)c1)C(=O)c1ccc([nH]1)-c1ccccc1 Show InChI InChI=1S/C34H46N4O4/c1-5-37(6-2)22-13-23-38(34(40)29-20-19-28(36-29)25-14-9-7-10-15-25)32(33(39)35-27-16-11-8-12-17-27)26-18-21-30(41-3)31(24-26)42-4/h7,9-10,14-15,18-21,24,27,32,36H,5-6,8,11-13,16-17,22-23H2,1-4H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186366
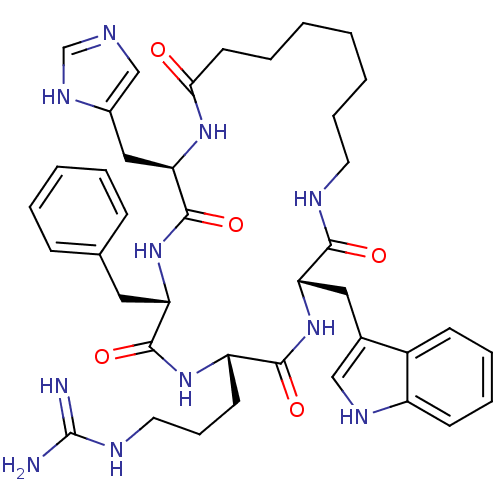
(CHEMBL211131 | c(his-L-phe-arg-trp-Aoc))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C40H53N11O5/c41-40(42)45-19-11-16-31-37(54)51-33(21-27-23-46-30-15-9-8-14-29(27)30)36(53)44-18-10-3-1-2-7-17-35(52)48-34(22-28-24-43-25-47-28)39(56)50-32(38(55)49-31)20-26-12-5-4-6-13-26/h4-6,8-9,12-15,23-25,31-34,46H,1-3,7,10-11,16-22H2,(H,43,47)(H,44,53)(H,48,52)(H,49,55)(H,50,56)(H,51,54)(H4,41,42,45)/t31-,32-,33-,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human cloned MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186368
(CHEMBL379531 | c(his-D-phe-arg-trp-Abu))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:7.6,38.40,(-8.86,-41.12,;-7.56,-41.93,;-7.61,-43.48,;-6.2,-41.21,;-4.89,-42.02,;-3.53,-41.29,;-2.22,-42.11,;-.86,-41.38,;-.73,-39.86,;-2.17,-39.31,;-3.43,-40.18,;-2.17,-37.78,;-3.43,-36.9,;-4.82,-37.56,;-6.09,-36.68,;-7.48,-37.33,;-7.61,-38.87,;-6.33,-39.75,;-4.94,-39.09,;-.73,-37.24,;-.85,-35.72,;-2.2,-34.97,;.51,-35.02,;.66,-33.49,;-.59,-32.59,;-2.05,-33.09,;-2.97,-31.84,;-2.07,-30.59,;-.6,-31.06,;1.72,-35.99,;2.97,-35.05,;2.86,-33.51,;4.48,-35.94,;3.78,-37.34,;5.46,-38.93,;4.2,-39.96,;4.33,-41.47,;5.68,-42.21,;2.96,-42.17,;2.8,-43.7,;4.05,-44.6,;5.51,-44.12,;6.43,-45.35,;5.53,-46.61,;5.86,-48.1,;4.72,-49.13,;3.25,-48.67,;2.94,-47.16,;4.07,-46.14,;1.73,-41.19,;.5,-42.12,;.61,-43.66,)| Show InChI InChI=1S/C36H45N11O5/c37-36(38)41-15-6-12-27-33(50)47-29(17-23-19-42-26-11-5-4-10-25(23)26)32(49)40-14-7-13-31(48)44-30(18-24-20-39-21-43-24)35(52)46-28(34(51)45-27)16-22-8-2-1-3-9-22/h1-5,8-11,19-21,27-30,42H,6-7,12-18H2,(H,39,43)(H,40,49)(H,44,48)(H,45,51)(H,46,52)(H,47,50)(H4,37,38,41)/t27-,28+,29-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human cloned MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50151162
((S)-3-(2-Chloro-phenyl)-1-(2-diethylamino-ethyl)-3...)Show SMILES CCN(CC)CCN1C(=O)[C@](O)(c2c1cc(cc2C(F)(F)F)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C22H23ClF3N3O3/c1-3-28(4-2)9-10-29-17-12-13(19(27)30)11-15(22(24,25)26)18(17)21(32,20(29)31)14-7-5-6-8-16(14)23/h5-8,11-12,32H,3-4,9-10H2,1-2H3,(H2,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Life Science Research
Curated by ChEMBL
| Assay Description
Binding affinity for human growth hormone secretagogue receptor was determined using [125I]-ghrelin |
J Med Chem 47: 4286-90 (2004)
Article DOI: 10.1021/jm040103i
BindingDB Entry DOI: 10.7270/Q2CR5SV7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304254
((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC1=O |r| Show InChI InChI=1S/C42H50N8O8/c43-18-4-3-8-32-38(54)49-35(21-26-12-16-29(52)17-13-26)42(58)50-19-5-9-36(50)41(57)45-24-37(53)46-33(20-25-10-14-28(51)15-11-25)39(55)48-34(40(56)47-32)22-27-23-44-31-7-2-1-6-30(27)31/h1-2,6-7,10-17,23,32-36,44,51-52H,3-5,8-9,18-22,24,43H2,(H,45,57)(H,46,53)(H,47,56)(H,48,55)(H,49,54)/t32-,33-,34-,35-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304255
((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C42H50N8O6/c43-20-10-9-18-32-38(52)49-35(23-28-14-5-2-6-15-28)42(56)50-21-11-19-36(50)41(55)45-26-37(51)46-33(22-27-12-3-1-4-13-27)39(53)48-34(40(54)47-32)24-29-25-44-31-17-8-7-16-30(29)31/h1-8,12-17,25,32-36,44H,9-11,18-24,26,43H2,(H,45,55)(H,46,51)(H,47,54)(H,48,53)(H,49,52)/t32-,33-,34-,35-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304256

((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C36H46N8O7/c37-15-7-6-13-26-32(47)43-28(17-22-9-2-1-3-10-22)36(51)44-16-8-14-30(44)35(50)39-20-31(46)40-29(21-45)34(49)42-27(33(48)41-26)18-23-19-38-25-12-5-4-11-24(23)25/h1-5,9-12,19,26-30,38,45H,6-8,13-18,20-21,37H2,(H,39,50)(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t26-,27-,28-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304257
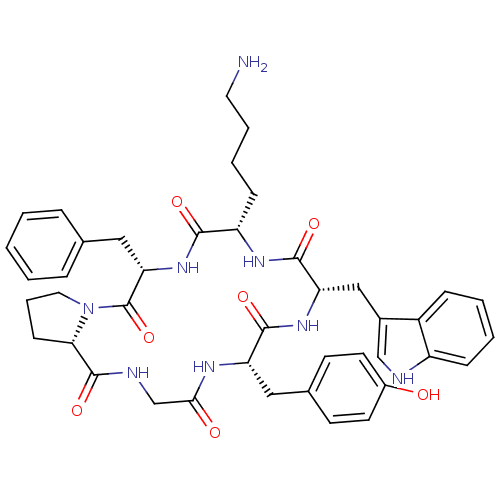
((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C42H50N8O7/c43-19-7-6-13-32-38(53)49-35(22-26-9-2-1-3-10-26)42(57)50-20-8-14-36(50)41(56)45-25-37(52)46-33(21-27-15-17-29(51)18-16-27)39(54)48-34(40(55)47-32)23-28-24-44-31-12-5-4-11-30(28)31/h1-5,9-12,15-18,24,32-36,44,51H,6-8,13-14,19-23,25,43H2,(H,45,56)(H,46,52)(H,47,55)(H,48,54)(H,49,53)/t32-,33-,34-,35-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 75.7 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304258
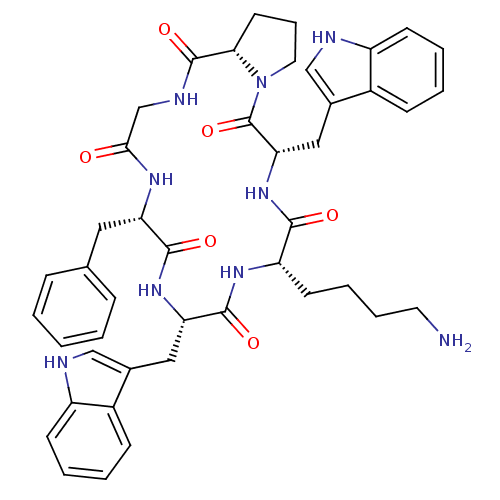
((6S,9S,12S,15S,20aS)-9,15-bis((1H-indol-3-yl)methy...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C44H51N9O6/c45-19-9-8-17-34-40(55)52-37(23-29-25-47-33-16-7-5-14-31(29)33)44(59)53-20-10-18-38(53)43(58)48-26-39(54)49-35(21-27-11-2-1-3-12-27)41(56)51-36(42(57)50-34)22-28-24-46-32-15-6-4-13-30(28)32/h1-7,11-16,24-25,34-38,46-47H,8-10,17-23,26,45H2,(H,48,58)(H,49,54)(H,50,57)(H,51,56)(H,52,55)/t34-,35-,36-,37-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20.6 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304253

((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC1=O |r| Show InChI InChI=1S/C36H46N8O8/c37-14-4-3-8-26-32(48)43-28(16-21-10-12-23(46)13-11-21)36(52)44-15-5-9-30(44)35(51)39-19-31(47)40-29(20-45)34(50)42-27(33(49)41-26)17-22-18-38-25-7-2-1-6-24(22)25/h1-2,6-7,10-13,18,26-30,38,45-46H,3-5,8-9,14-17,19-20,37H2,(H,39,51)(H,40,47)(H,41,49)(H,42,50)(H,43,48)/t26-,27-,28-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304252

((6S,9S,12S,15S,20aS)-9-((1H-indol-3-yl)methyl)-12-...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC1=O |r| Show InChI InChI=1S/C42H50N8O7/c43-19-7-6-13-32-38(53)49-35(22-27-15-17-29(51)18-16-27)42(57)50-20-8-14-36(50)41(56)45-25-37(52)46-33(21-26-9-2-1-3-10-26)39(54)48-34(40(55)47-32)23-28-24-44-31-12-5-4-11-30(28)31/h1-5,9-12,15-18,24,32-36,44,51H,6-8,13-14,19-23,25,43H2,(H,45,56)(H,46,52)(H,47,55)(H,48,54)(H,49,53)/t32-,33-,34-,35-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.94 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50378580
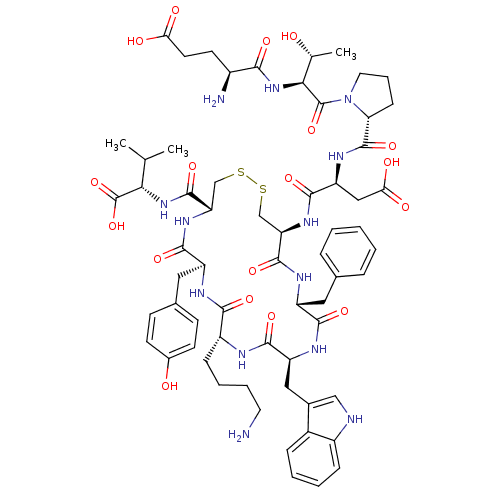
(CHEMBL437430)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C64H85N13O18S2/c1-33(2)52(64(94)95)75-61(91)48-32-97-96-31-47(73-59(89)46(29-51(82)83)72-62(92)49-17-11-25-77(49)63(93)53(34(3)78)76-54(84)40(66)22-23-50(80)81)60(90)70-43(26-35-12-5-4-6-13-35)56(86)71-45(28-37-30-67-41-15-8-7-14-39(37)41)58(88)68-42(16-9-10-24-65)55(85)69-44(57(87)74-48)27-36-18-20-38(79)21-19-36/h4-8,12-15,18-21,30,33-34,40,42-49,52-53,67,78-79H,9-11,16-17,22-29,31-32,65-66H2,1-3H3,(H,68,88)(H,69,85)(H,70,90)(H,71,86)(H,72,92)(H,73,89)(H,74,87)(H,75,91)(H,76,84)(H,80,81)(H,82,83)(H,94,95)/t34-,40+,42-,43-,44+,45+,46+,47-,48+,49-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186371

(CHEMBL264120 | c(his-D-phe-arg-trp-Aoc))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC[C@H](Cc2cnc[nH]2)NC(=O)CCCCCCCC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:21.22,40.42,7.6,wD:11.11,(14.11,-13.67,;13.11,-12.52,;13.62,-11.08,;11.62,-12.8,;10.62,-11.65,;9.13,-11.93,;8.13,-10.78,;6.64,-11.06,;5.6,-9.87,;4.18,-9.13,;4.11,-7.53,;2.85,-9.99,;2.84,-8.38,;1.44,-7.58,;1.43,-5.97,;.03,-5.18,;-1.36,-5.99,;-1.33,-7.78,;.06,-8.4,;1.47,-10.67,;.04,-10.09,;-1.33,-9.3,;-2.1,-7.97,;-3.62,-7.97,;-4.53,-6.74,;-5.97,-7.21,;-5.97,-8.73,;-4.52,-9.2,;-2.59,-10.19,;-2.67,-11.73,;-4.47,-12.71,;-1.28,-12.48,;-1.28,-14.08,;.11,-14.88,;1.49,-14.07,;2.87,-14.87,;2.87,-16.48,;4.47,-17.01,;5.02,-15.59,;4.11,-14.39,;6.58,-15.28,;7.56,-16.44,;7.05,-17.88,;5.59,-18.3,;5.55,-19.83,;6.98,-20.34,;7.55,-21.74,;9.06,-21.95,;9.99,-20.74,;9.41,-19.34,;7.91,-19.14,;7.02,-13.81,;6.1,-12.57,;4.53,-12.87,)| Show InChI InChI=1S/C40H54N10O4/c41-40(42)44-19-11-16-33-38(53)50-34(21-28-23-45-32-15-10-9-14-31(28)32)36(51)17-7-2-1-3-8-18-37(52)48-30(22-29-24-43-26-47-29)25-46-35(39(54)49-33)20-27-12-5-4-6-13-27/h4-6,9-10,12-15,23-24,26,30,33-35,45-46H,1-3,7-8,11,16-22,25H2,(H,43,47)(H,48,52)(H,49,54)(H,50,53)(H4,41,42,44)/t30-,33-,34-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 77.4 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186367

(CHEMBL379959 | c(his-D-phe-arg-trp-Ahp))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C39H51N11O5/c40-39(41)44-18-10-15-30-36(53)50-32(20-26-22-45-29-14-8-7-13-28(26)29)35(52)43-17-9-2-1-6-16-34(51)47-33(21-27-23-42-24-46-27)38(55)49-31(37(54)48-30)19-25-11-4-3-5-12-25/h3-5,7-8,11-14,22-24,30-33,45H,1-2,6,9-10,15-21H2,(H,42,46)(H,43,52)(H,47,51)(H,48,54)(H,49,55)(H,50,53)(H4,40,41,44)/t30-,31+,32+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186370

(CHEMBL379627 | c(his-D-phe-arg-trp-Ahx))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:40.42,7.6,(.29,-13.95,;1.12,-12.66,;.41,-11.3,;2.65,-12.73,;3.47,-11.44,;2.77,-10.08,;3.6,-8.8,;2.89,-7.42,;1.34,-6.07,;-.11,-7.87,;-.39,-9.37,;-1.55,-7.32,;-2.32,-8.66,;-3.85,-8.66,;-4.63,-10,;-6.16,-10,;-6.92,-8.68,;-6.16,-7.36,;-4.63,-7.36,;-1.93,-5.49,;-3.54,-5.03,;-4.98,-5.63,;-3.9,-3.54,;-5.44,-3.64,;-6.35,-2.39,;-7.88,-2.39,;-8.36,-.92,;-7.11,-.03,;-5.87,-.92,;-2.4,-2.19,;-3.11,-.61,;-4.37,.26,;-2.02,.48,;-.16,-.65,;.87,1.42,;1.85,-.18,;3.71,.36,;3.41,-1.72,;5.32,-2.22,;6.82,-1.87,;5.42,-3.75,;6.94,-3.91,;7.57,-5.32,;6.8,-6.65,;7.83,-7.8,;9.23,-7.17,;10.64,-7.8,;11.89,-6.89,;11.72,-5.35,;10.32,-4.73,;9.08,-5.63,;3.46,-4.56,;4.14,-6.49,;5.23,-7.58,)| Show InChI InChI=1S/C38H49N11O5/c39-38(40)43-17-9-14-29-35(52)49-31(19-25-21-44-28-13-7-6-12-27(25)28)34(51)42-16-8-2-5-15-33(50)46-32(20-26-22-41-23-45-26)37(54)48-30(36(53)47-29)18-24-10-3-1-4-11-24/h1,3-4,6-7,10-13,21-23,29-32,44H,2,5,8-9,14-20H2,(H,41,45)(H,42,51)(H,46,50)(H,47,53)(H,48,54)(H,49,52)(H4,39,40,43)/t29-,30+,31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747
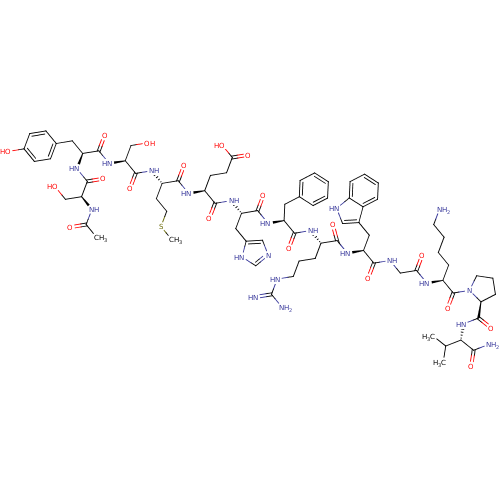
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 72.9 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186370

(CHEMBL379627 | c(his-D-phe-arg-trp-Ahx))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:40.42,7.6,(.29,-13.95,;1.12,-12.66,;.41,-11.3,;2.65,-12.73,;3.47,-11.44,;2.77,-10.08,;3.6,-8.8,;2.89,-7.42,;1.34,-6.07,;-.11,-7.87,;-.39,-9.37,;-1.55,-7.32,;-2.32,-8.66,;-3.85,-8.66,;-4.63,-10,;-6.16,-10,;-6.92,-8.68,;-6.16,-7.36,;-4.63,-7.36,;-1.93,-5.49,;-3.54,-5.03,;-4.98,-5.63,;-3.9,-3.54,;-5.44,-3.64,;-6.35,-2.39,;-7.88,-2.39,;-8.36,-.92,;-7.11,-.03,;-5.87,-.92,;-2.4,-2.19,;-3.11,-.61,;-4.37,.26,;-2.02,.48,;-.16,-.65,;.87,1.42,;1.85,-.18,;3.71,.36,;3.41,-1.72,;5.32,-2.22,;6.82,-1.87,;5.42,-3.75,;6.94,-3.91,;7.57,-5.32,;6.8,-6.65,;7.83,-7.8,;9.23,-7.17,;10.64,-7.8,;11.89,-6.89,;11.72,-5.35,;10.32,-4.73,;9.08,-5.63,;3.46,-4.56,;4.14,-6.49,;5.23,-7.58,)| Show InChI InChI=1S/C38H49N11O5/c39-38(40)43-17-9-14-29-35(52)49-31(19-25-21-44-28-13-7-6-12-27(25)28)34(51)42-16-8-2-5-15-33(50)46-32(20-26-22-41-23-45-26)37(54)48-30(36(53)47-29)18-24-10-3-1-4-11-24/h1,3-4,6-7,10-13,21-23,29-32,44H,2,5,8-9,14-20H2,(H,41,45)(H,42,51)(H,46,50)(H,47,53)(H,48,54)(H,49,52)(H4,39,40,43)/t29-,30+,31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186368

(CHEMBL379531 | c(his-D-phe-arg-trp-Abu))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:7.6,38.40,(-8.86,-41.12,;-7.56,-41.93,;-7.61,-43.48,;-6.2,-41.21,;-4.89,-42.02,;-3.53,-41.29,;-2.22,-42.11,;-.86,-41.38,;-.73,-39.86,;-2.17,-39.31,;-3.43,-40.18,;-2.17,-37.78,;-3.43,-36.9,;-4.82,-37.56,;-6.09,-36.68,;-7.48,-37.33,;-7.61,-38.87,;-6.33,-39.75,;-4.94,-39.09,;-.73,-37.24,;-.85,-35.72,;-2.2,-34.97,;.51,-35.02,;.66,-33.49,;-.59,-32.59,;-2.05,-33.09,;-2.97,-31.84,;-2.07,-30.59,;-.6,-31.06,;1.72,-35.99,;2.97,-35.05,;2.86,-33.51,;4.48,-35.94,;3.78,-37.34,;5.46,-38.93,;4.2,-39.96,;4.33,-41.47,;5.68,-42.21,;2.96,-42.17,;2.8,-43.7,;4.05,-44.6,;5.51,-44.12,;6.43,-45.35,;5.53,-46.61,;5.86,-48.1,;4.72,-49.13,;3.25,-48.67,;2.94,-47.16,;4.07,-46.14,;1.73,-41.19,;.5,-42.12,;.61,-43.66,)| Show InChI InChI=1S/C36H45N11O5/c37-36(38)41-15-6-12-27-33(50)47-29(17-23-19-42-26-11-5-4-10-25(23)26)32(49)40-14-7-13-31(48)44-30(18-24-20-39-21-43-24)35(52)46-28(34(51)45-27)16-22-8-2-1-3-9-22/h1-5,8-11,19-21,27-30,42H,6-7,12-18H2,(H,39,43)(H,40,49)(H,44,48)(H,45,51)(H,46,52)(H,47,50)(H4,37,38,41)/t27-,28+,29-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186372
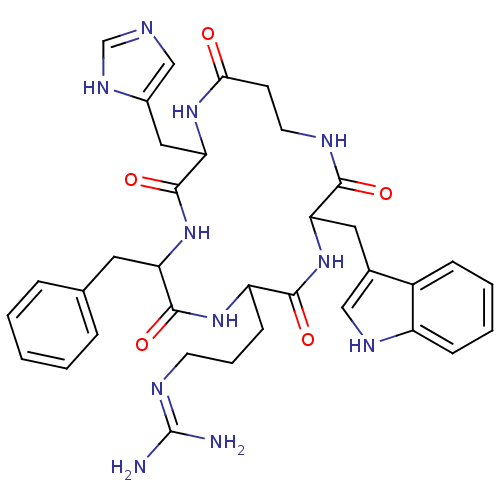
(CHEMBL380198 | c(his-D-phe-arg-trp-betaala))Show SMILES NC(N)=NCCCC1NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2cnc[nH]2)NC(=O)CCNC(=O)C(Cc2c[nH]c3ccccc23)NC1=O |(6.52,-19.81,;7.85,-20.58,;7.85,-22.12,;9.18,-19.81,;10.52,-20.59,;11.85,-19.82,;13.18,-20.59,;14.52,-19.82,;14.52,-18.25,;13.18,-17.48,;11.84,-18.24,;13.2,-16.03,;11.87,-15.25,;10.53,-16.01,;9.21,-15.22,;7.87,-15.98,;7.86,-17.52,;9.19,-18.3,;10.53,-17.54,;14.54,-15.26,;15.86,-16.03,;15.85,-17.57,;17.22,-15.27,;17.21,-13.73,;15.88,-12.96,;14.49,-13.59,;13.45,-12.45,;14.21,-11.11,;15.72,-11.43,;18.58,-16.03,;19.91,-15.25,;19.9,-13.71,;21.26,-16.01,;21.28,-17.48,;19.95,-18.25,;19.95,-19.82,;21.28,-20.59,;18.59,-20.6,;18.59,-22.14,;19.92,-22.91,;21.32,-22.29,;22.35,-23.43,;21.59,-24.77,;22.06,-26.22,;21.04,-27.36,;19.53,-27.05,;19.06,-25.59,;20.08,-24.45,;17.23,-19.82,;15.86,-20.6,;15.86,-22.14,)| Show InChI InChI=1S/C35H43N11O5/c36-35(37)40-13-6-11-26-32(49)46-28(16-22-18-41-25-10-5-4-9-24(22)25)31(48)39-14-12-30(47)43-29(17-23-19-38-20-42-23)34(51)45-27(33(50)44-26)15-21-7-2-1-3-8-21/h1-5,7-10,18-20,26-29,41H,6,11-17H2,(H,38,42)(H,39,48)(H,43,47)(H,44,50)(H,45,51)(H,46,49)(H4,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186367

(CHEMBL379959 | c(his-D-phe-arg-trp-Ahp))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C39H51N11O5/c40-39(41)44-18-10-15-30-36(53)50-32(20-26-22-45-29-14-8-7-13-28(26)29)35(52)43-17-9-2-1-6-16-34(51)47-33(21-27-23-42-24-46-27)38(55)49-31(37(54)48-30)19-25-11-4-3-5-12-25/h3-5,7-8,11-14,22-24,30-33,45H,1-2,6,9-10,15-21H2,(H,42,46)(H,43,52)(H,47,51)(H,48,54)(H,49,55)(H,50,53)(H4,40,41,44)/t30-,31+,32+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15.4 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186372

(CHEMBL380198 | c(his-D-phe-arg-trp-betaala))Show SMILES NC(N)=NCCCC1NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2cnc[nH]2)NC(=O)CCNC(=O)C(Cc2c[nH]c3ccccc23)NC1=O |(6.52,-19.81,;7.85,-20.58,;7.85,-22.12,;9.18,-19.81,;10.52,-20.59,;11.85,-19.82,;13.18,-20.59,;14.52,-19.82,;14.52,-18.25,;13.18,-17.48,;11.84,-18.24,;13.2,-16.03,;11.87,-15.25,;10.53,-16.01,;9.21,-15.22,;7.87,-15.98,;7.86,-17.52,;9.19,-18.3,;10.53,-17.54,;14.54,-15.26,;15.86,-16.03,;15.85,-17.57,;17.22,-15.27,;17.21,-13.73,;15.88,-12.96,;14.49,-13.59,;13.45,-12.45,;14.21,-11.11,;15.72,-11.43,;18.58,-16.03,;19.91,-15.25,;19.9,-13.71,;21.26,-16.01,;21.28,-17.48,;19.95,-18.25,;19.95,-19.82,;21.28,-20.59,;18.59,-20.6,;18.59,-22.14,;19.92,-22.91,;21.32,-22.29,;22.35,-23.43,;21.59,-24.77,;22.06,-26.22,;21.04,-27.36,;19.53,-27.05,;19.06,-25.59,;20.08,-24.45,;17.23,-19.82,;15.86,-20.6,;15.86,-22.14,)| Show InChI InChI=1S/C35H43N11O5/c36-35(37)40-13-6-11-26-32(49)46-28(16-22-18-41-25-10-5-4-9-24(22)25)31(48)39-14-12-30(47)43-29(17-23-19-38-20-42-23)34(51)45-27(33(50)44-26)15-21-7-2-1-3-8-21/h1-5,7-10,18-20,26-29,41H,6,11-17H2,(H,38,42)(H,39,48)(H,43,47)(H,44,50)(H,45,51)(H,46,49)(H4,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186369
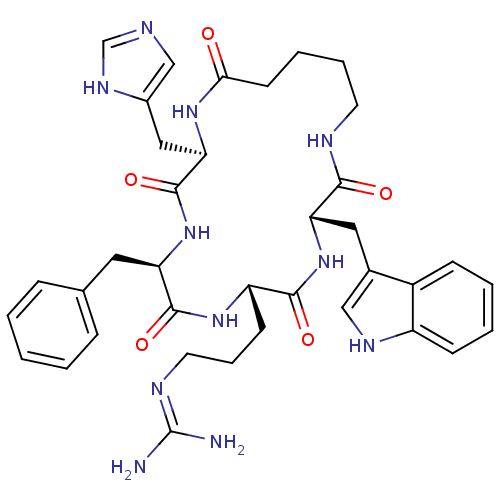
(CHEMBL210399 | c(his-D-phe-arg-trp-Ava))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:7.6,39.41,(2.76,-12.78,;4.16,-12.13,;4.28,-10.59,;5.42,-13,;6.81,-12.35,;8.08,-13.22,;7.95,-14.76,;9.22,-15.63,;10.66,-15.1,;10.53,-13.57,;9.19,-12.82,;11.9,-12.88,;12.05,-11.34,;10.8,-10.45,;10.96,-8.91,;9.71,-8.01,;8.3,-8.65,;8.15,-10.19,;9.41,-11.08,;13.12,-13.84,;14.37,-12.9,;14.26,-11.37,;15.74,-13.65,;17.1,-12.92,;17.15,-11.38,;15.94,-10.44,;16.46,-8.99,;18,-9.03,;18.43,-10.52,;15.61,-15.17,;17.06,-15.73,;18.33,-14.86,;17.06,-17.26,;15.61,-17.82,;15.74,-19.34,;14.36,-20.04,;13.13,-19.07,;11.89,-19.99,;12,-21.53,;10.52,-19.26,;9.17,-19.98,;9.12,-21.52,;10.33,-22.46,;9.81,-23.91,;8.27,-23.87,;7.21,-24.97,;5.72,-24.61,;5.29,-23.13,;6.36,-22.02,;7.85,-22.39,;10.66,-17.73,;9.22,-17.17,;7.95,-18.03,)| Show InChI InChI=1S/C37H47N11O5/c38-37(39)42-16-8-13-28-34(51)48-30(18-24-20-43-27-12-5-4-11-26(24)27)33(50)41-15-7-6-14-32(49)45-31(19-25-21-40-22-44-25)36(53)47-29(35(52)46-28)17-23-9-2-1-3-10-23/h1-5,9-12,20-22,28-31,43H,6-8,13-19H2,(H,40,44)(H,41,50)(H,45,49)(H,46,52)(H,47,53)(H,48,51)(H4,38,39,42)/t28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50304259
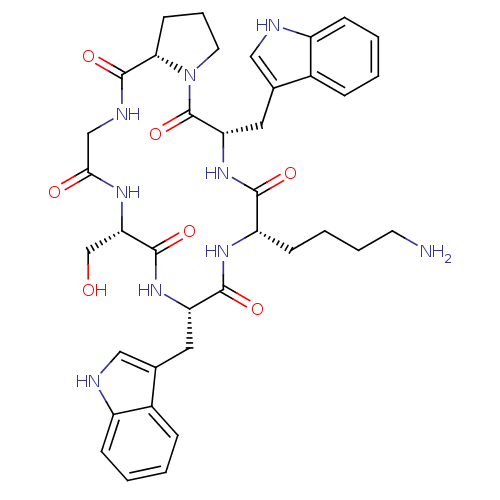
((6S,9S,12S,15S,20aS)-9,15-bis((1H-indol-3-yl)methy...)Show SMILES NCCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C38H47N9O7/c39-14-6-5-12-28-34(50)46-30(17-23-19-41-27-11-4-2-9-25(23)27)38(54)47-15-7-13-32(47)37(53)42-20-33(49)43-31(21-48)36(52)45-29(35(51)44-28)16-22-18-40-26-10-3-1-8-24(22)26/h1-4,8-11,18-19,28-32,40-41,48H,5-7,12-17,20-21,39H2,(H,42,53)(H,43,49)(H,44,51)(H,45,52)(H,46,50)/t28-,29-,30-,31-,32-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a |
PRISM Bio. Lab. Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR14 expressed in HEK293 cells by calcium mobilization assay |
Bioorg Med Chem 17: 6742-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.058
BindingDB Entry DOI: 10.7270/Q2F47Q28 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186369

(CHEMBL210399 | c(his-D-phe-arg-trp-Ava))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:7.6,39.41,(2.76,-12.78,;4.16,-12.13,;4.28,-10.59,;5.42,-13,;6.81,-12.35,;8.08,-13.22,;7.95,-14.76,;9.22,-15.63,;10.66,-15.1,;10.53,-13.57,;9.19,-12.82,;11.9,-12.88,;12.05,-11.34,;10.8,-10.45,;10.96,-8.91,;9.71,-8.01,;8.3,-8.65,;8.15,-10.19,;9.41,-11.08,;13.12,-13.84,;14.37,-12.9,;14.26,-11.37,;15.74,-13.65,;17.1,-12.92,;17.15,-11.38,;15.94,-10.44,;16.46,-8.99,;18,-9.03,;18.43,-10.52,;15.61,-15.17,;17.06,-15.73,;18.33,-14.86,;17.06,-17.26,;15.61,-17.82,;15.74,-19.34,;14.36,-20.04,;13.13,-19.07,;11.89,-19.99,;12,-21.53,;10.52,-19.26,;9.17,-19.98,;9.12,-21.52,;10.33,-22.46,;9.81,-23.91,;8.27,-23.87,;7.21,-24.97,;5.72,-24.61,;5.29,-23.13,;6.36,-22.02,;7.85,-22.39,;10.66,-17.73,;9.22,-17.17,;7.95,-18.03,)| Show InChI InChI=1S/C37H47N11O5/c38-37(39)42-16-8-13-28-34(51)48-30(18-24-20-43-27-12-5-4-11-26(24)27)33(50)41-15-7-6-14-32(49)45-31(19-25-21-40-22-44-25)36(53)47-29(35(52)46-28)17-23-9-2-1-3-10-23/h1-5,9-12,20-22,28-31,43H,6-8,13-19H2,(H,40,44)(H,41,50)(H,45,49)(H,46,52)(H,47,53)(H,48,51)(H4,38,39,42)/t28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186368

(CHEMBL379531 | c(his-D-phe-arg-trp-Abu))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:11.11,22.23,wD:7.6,38.40,(-8.86,-41.12,;-7.56,-41.93,;-7.61,-43.48,;-6.2,-41.21,;-4.89,-42.02,;-3.53,-41.29,;-2.22,-42.11,;-.86,-41.38,;-.73,-39.86,;-2.17,-39.31,;-3.43,-40.18,;-2.17,-37.78,;-3.43,-36.9,;-4.82,-37.56,;-6.09,-36.68,;-7.48,-37.33,;-7.61,-38.87,;-6.33,-39.75,;-4.94,-39.09,;-.73,-37.24,;-.85,-35.72,;-2.2,-34.97,;.51,-35.02,;.66,-33.49,;-.59,-32.59,;-2.05,-33.09,;-2.97,-31.84,;-2.07,-30.59,;-.6,-31.06,;1.72,-35.99,;2.97,-35.05,;2.86,-33.51,;4.48,-35.94,;3.78,-37.34,;5.46,-38.93,;4.2,-39.96,;4.33,-41.47,;5.68,-42.21,;2.96,-42.17,;2.8,-43.7,;4.05,-44.6,;5.51,-44.12,;6.43,-45.35,;5.53,-46.61,;5.86,-48.1,;4.72,-49.13,;3.25,-48.67,;2.94,-47.16,;4.07,-46.14,;1.73,-41.19,;.5,-42.12,;.61,-43.66,)| Show InChI InChI=1S/C36H45N11O5/c37-36(38)41-15-6-12-27-33(50)47-29(17-23-19-42-26-11-5-4-10-25(23)26)32(49)40-14-7-13-31(48)44-30(18-24-20-39-21-43-24)35(52)46-28(34(51)45-27)16-22-8-2-1-3-9-22/h1-5,8-11,19-21,27-30,42H,6-7,12-18H2,(H,39,43)(H,40,49)(H,44,48)(H,45,51)(H,46,52)(H,47,50)(H4,37,38,41)/t27-,28+,29-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186366

(CHEMBL211131 | c(his-L-phe-arg-trp-Aoc))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C40H53N11O5/c41-40(42)45-19-11-16-31-37(54)51-33(21-27-23-46-30-15-9-8-14-29(27)30)36(53)44-18-10-3-1-2-7-17-35(52)48-34(22-28-24-43-25-47-28)39(56)50-32(38(55)49-31)20-26-12-5-4-6-13-26/h4-6,8-9,12-15,23-25,31-34,46H,1-3,7,10-11,16-22H2,(H,43,47)(H,44,53)(H,48,52)(H,49,55)(H,50,56)(H,51,54)(H4,41,42,45)/t31-,32-,33-,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.59E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50186366

(CHEMBL211131 | c(his-L-phe-arg-trp-Aoc))Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)CCCCCCCNC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O Show InChI InChI=1S/C40H53N11O5/c41-40(42)45-19-11-16-31-37(54)51-33(21-27-23-46-30-15-9-8-14-29(27)30)36(53)44-18-10-3-1-2-7-17-35(52)48-34(22-28-24-43-25-47-28)39(56)50-32(38(55)49-31)20-26-12-5-4-6-13-26/h4-6,8-9,12-15,23-25,31-34,46H,1-3,7,10-11,16-22H2,(H,43,47)(H,44,53)(H,48,52)(H,49,55)(H,50,56)(H,51,54)(H4,41,42,45)/t31-,32-,33-,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC1R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186371

(CHEMBL264120 | c(his-D-phe-arg-trp-Aoc))Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC[C@H](Cc2cnc[nH]2)NC(=O)CCCCCCCC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |wU:21.22,40.42,7.6,wD:11.11,(14.11,-13.67,;13.11,-12.52,;13.62,-11.08,;11.62,-12.8,;10.62,-11.65,;9.13,-11.93,;8.13,-10.78,;6.64,-11.06,;5.6,-9.87,;4.18,-9.13,;4.11,-7.53,;2.85,-9.99,;2.84,-8.38,;1.44,-7.58,;1.43,-5.97,;.03,-5.18,;-1.36,-5.99,;-1.33,-7.78,;.06,-8.4,;1.47,-10.67,;.04,-10.09,;-1.33,-9.3,;-2.1,-7.97,;-3.62,-7.97,;-4.53,-6.74,;-5.97,-7.21,;-5.97,-8.73,;-4.52,-9.2,;-2.59,-10.19,;-2.67,-11.73,;-4.47,-12.71,;-1.28,-12.48,;-1.28,-14.08,;.11,-14.88,;1.49,-14.07,;2.87,-14.87,;2.87,-16.48,;4.47,-17.01,;5.02,-15.59,;4.11,-14.39,;6.58,-15.28,;7.56,-16.44,;7.05,-17.88,;5.59,-18.3,;5.55,-19.83,;6.98,-20.34,;7.55,-21.74,;9.06,-21.95,;9.99,-20.74,;9.41,-19.34,;7.91,-19.14,;7.02,-13.81,;6.1,-12.57,;4.53,-12.87,)| Show InChI InChI=1S/C40H54N10O4/c41-40(42)44-19-11-16-33-38(53)50-34(21-28-23-45-32-15-10-9-14-31(28)32)36(51)17-7-2-1-3-8-18-37(52)48-30(22-29-24-43-26-47-29)25-46-35(39(54)49-33)20-27-12-5-4-6-13-27/h4-6,9-10,12-15,23-24,26,30,33-35,45-46H,1-3,7-8,11,16-22,25H2,(H,43,47)(H,48,52)(H,49,54)(H,50,53)(H4,41,42,44)/t30-,33-,34-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Activity at human MC4R by cAMP accumulation in SaoS2 cells |
Bioorg Med Chem Lett 16: 3723-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.050
BindingDB Entry DOI: 10.7270/Q2N58N5C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data











































