

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase A (Streptococcus pneumoniae) | BDBM50182491 (CHEBI:69015 | Malabaricone C) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60 mi... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50182486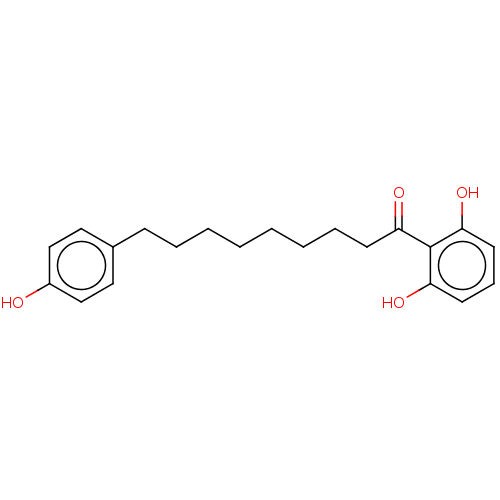 (MALABARICONE B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60 mi... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50303147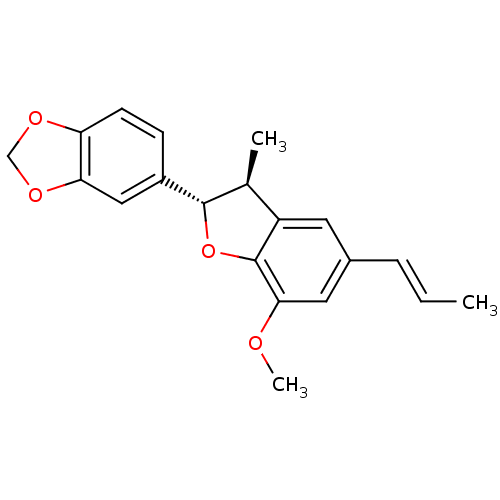 ((-)-licarin B | CHEMBL578403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259983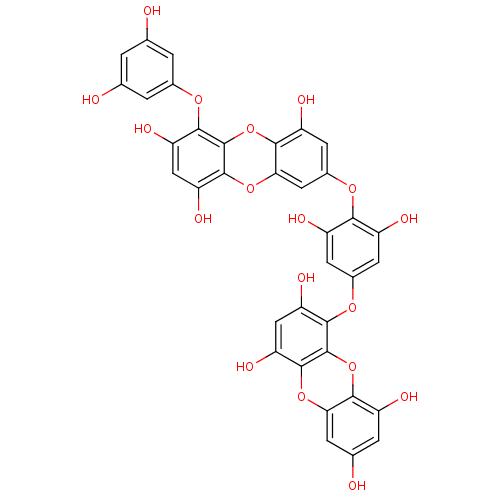 (CHEMBL508791 | US10106521, Compound Dieckol | diec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242962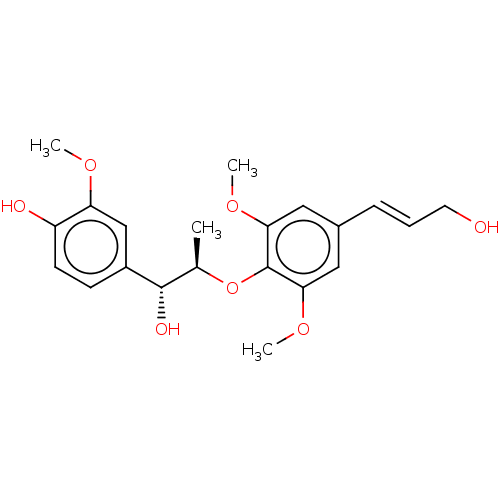 (CHEMBL4079519) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50157325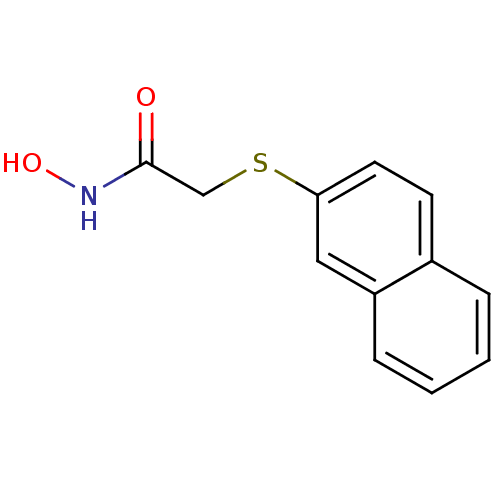 (CHEMBL182879 | N-Hydroxy-2-(naphthalen-2-ylsulfany...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity towards aminopeptidase N | Bioorg Med Chem Lett 15: 181-3 (2004) Article DOI: 10.1016/j.bmcl.2004.10.010 BindingDB Entry DOI: 10.7270/Q2BG2PRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242963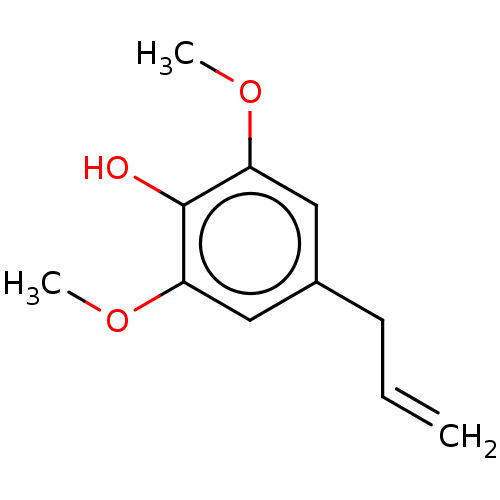 (CHEBI:86562 | Methoxyeugenol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242964 (CHEMBL4072450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50259982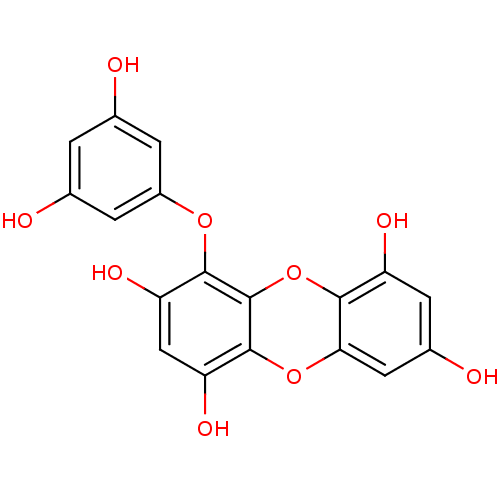 (CHEMBL471187 | US10106521, Compound Eckol | eckol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM53072 ((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50303149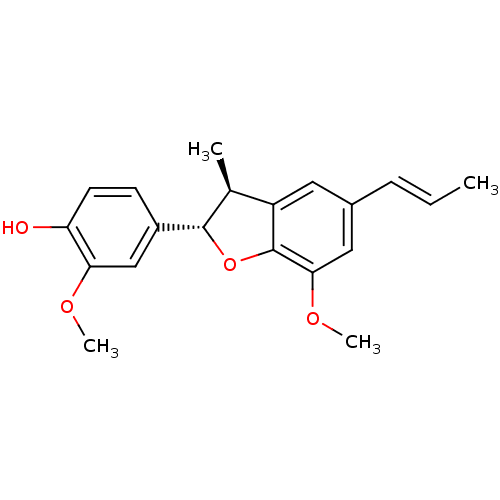 ((-)-licarin A | CHEMBL463526 | Licarin A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391431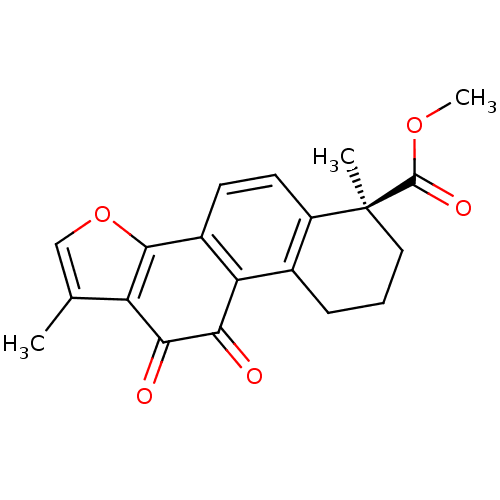 (CHEMBL2146517 | acs.jmedchem.1c00409_ST.502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50276756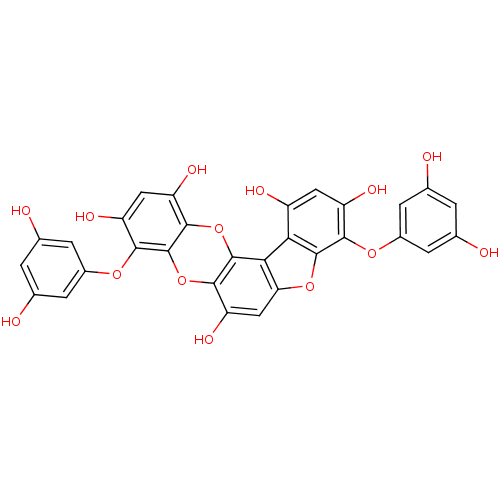 (4,9-bis(3,5-dihydroxyphenoxy)benzofuro[3,2-a]diben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM83922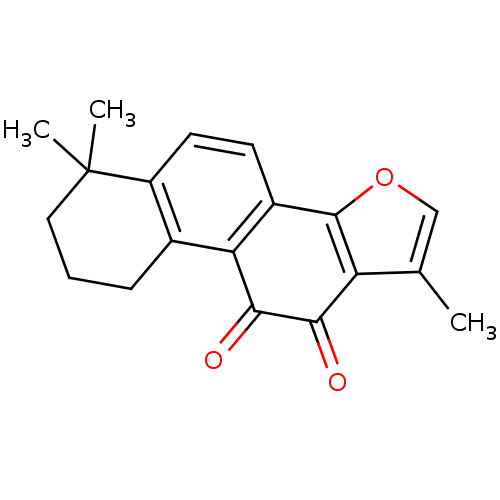 (1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50423877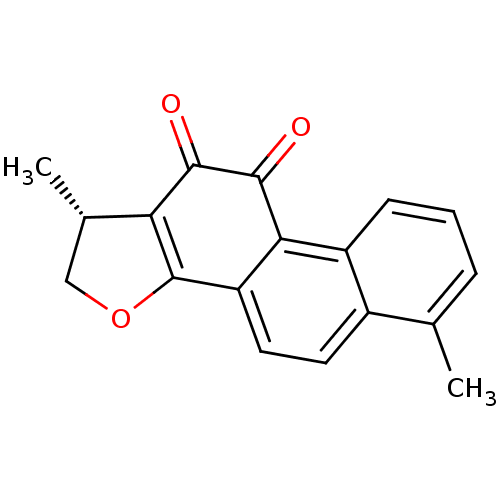 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM51317 (1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dion...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242967 (CHEMBL4085435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50276826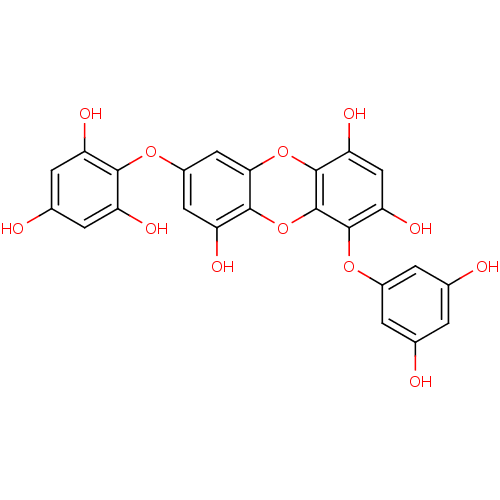 ((1-(3',5'-dihydroxyphenoxy)-7-(2'',4'',6-trihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50009219 (2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391429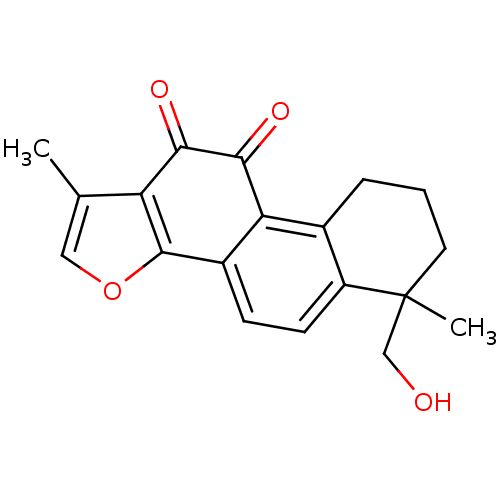 (CHEMBL215254 | acs.jmedchem.1c00409_ST.620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242959 (CHEMBL3601519) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50491981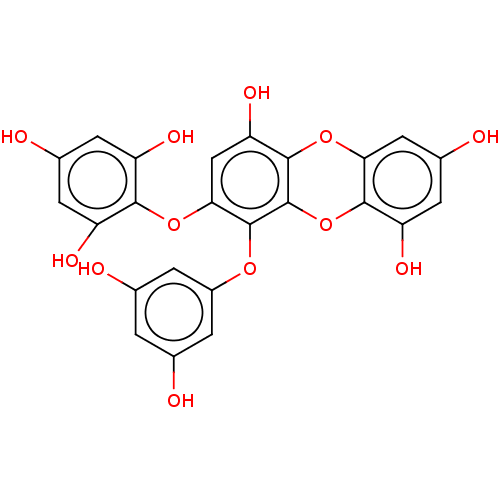 (2-PHLOROECKOL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase A (Streptococcus pneumoniae) | BDBM50242961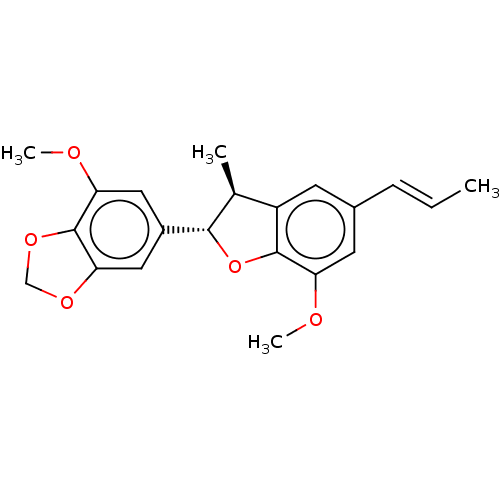 (CHEMBL4091275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Streptococcus pneumoniae sialidase NanA using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate after 60... | Bioorg Med Chem Lett 27: 3060-3064 (2017) Article DOI: 10.1016/j.bmcl.2017.05.055 BindingDB Entry DOI: 10.7270/Q2G73H5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50491983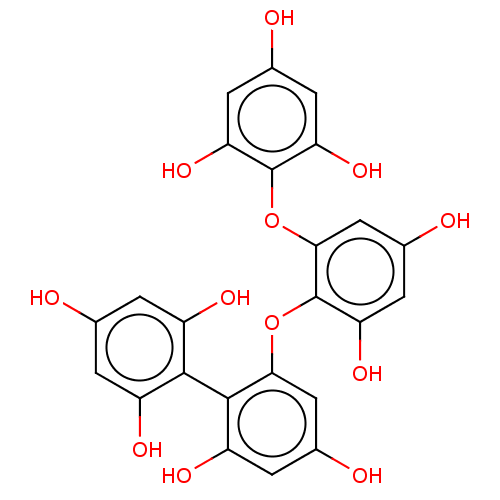 (CHEBI:65918 | Fucodiphloroethol G) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Competitive inhibition of C-terminal His6-tagged recombinant SARS coronavirus 3C-like protease trans-cleavage activity expressed in Escherichia coli ... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489360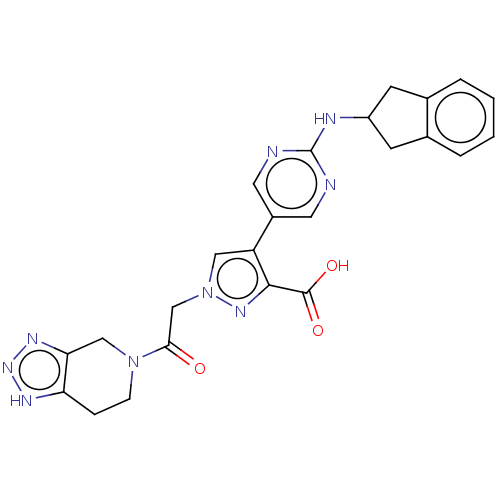 (Example 10-15 | US10961242, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343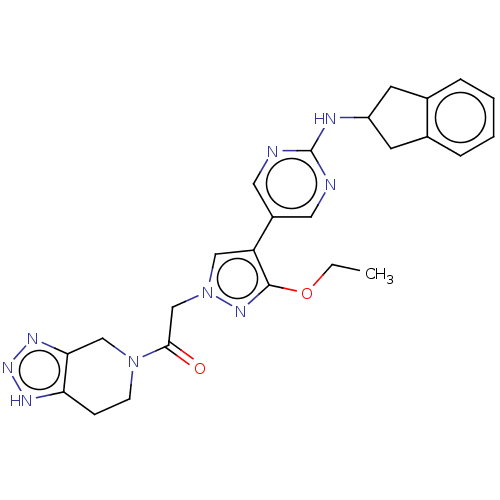 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582922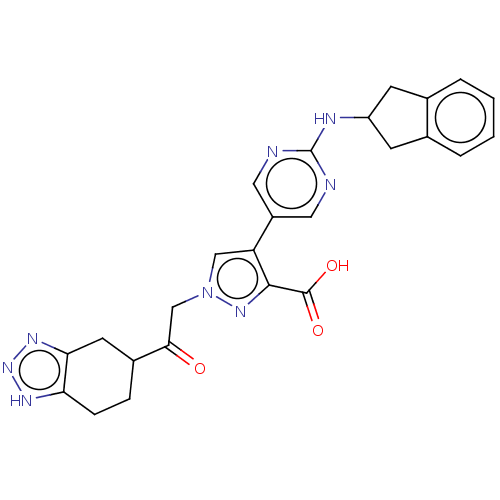 (Acid | US11548883, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367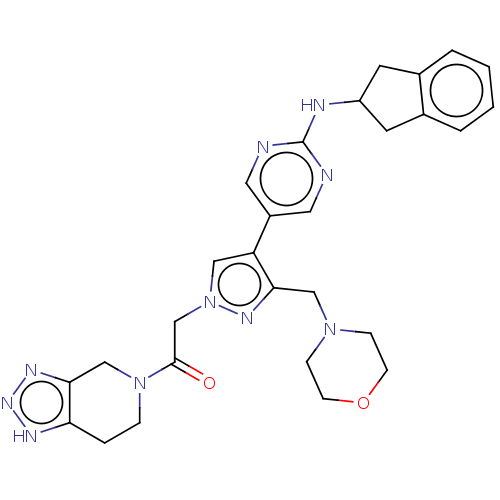 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368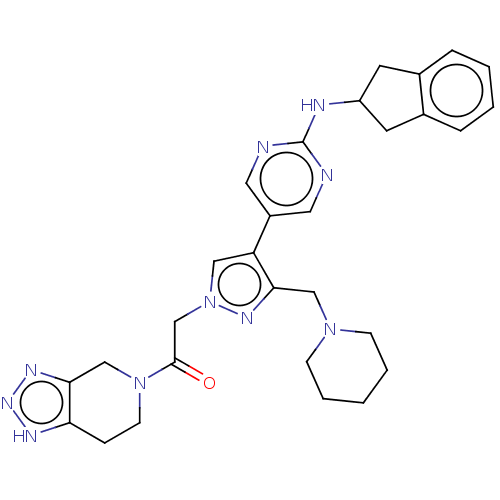 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347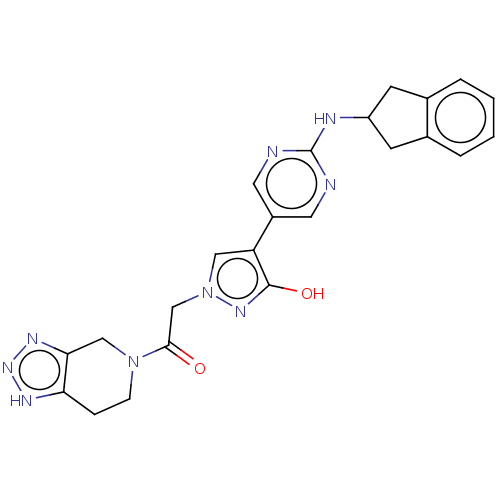 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369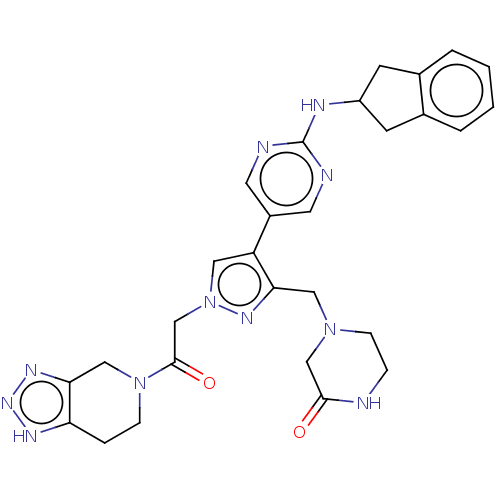 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366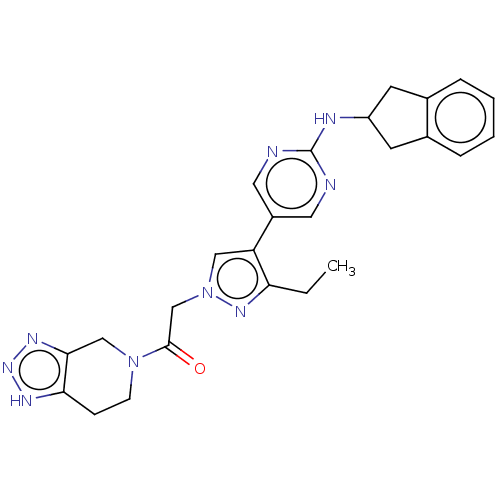 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357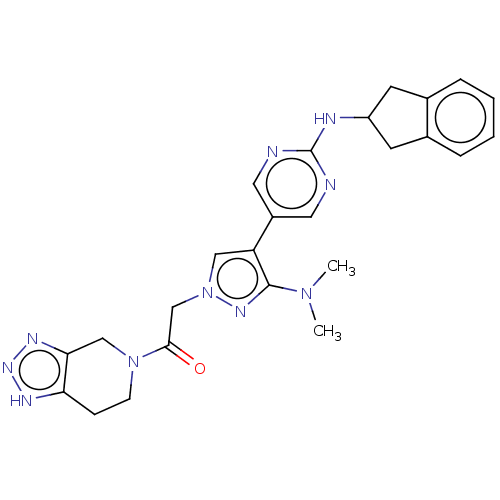 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582907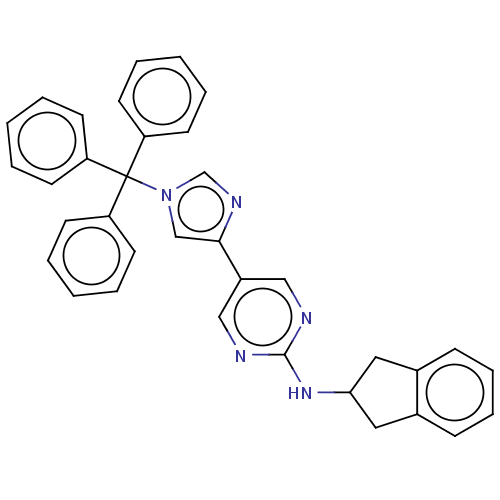 (2-(4-{2-[(2,3-dihydro-1H-inden-2-yl)amino]pyrimidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489342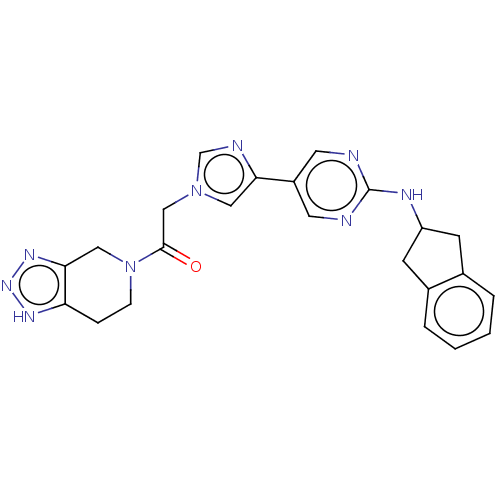 (Example 9 | US10961242, Compound 60) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351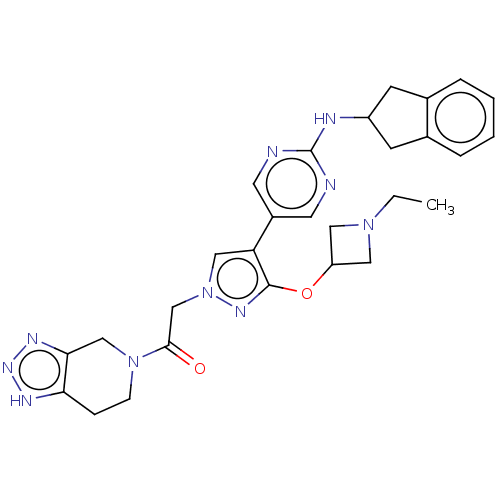 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348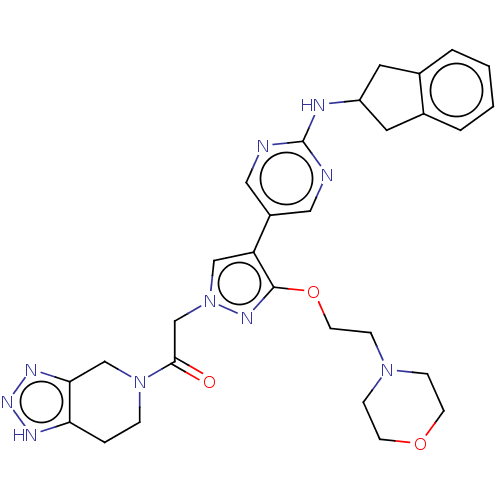 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489337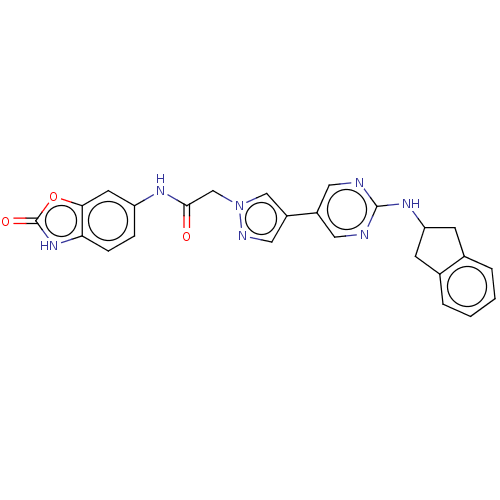 (Example 8-2 | US10961242, Compound 57 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489337 (Example 8-2 | US10961242, Compound 57 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489315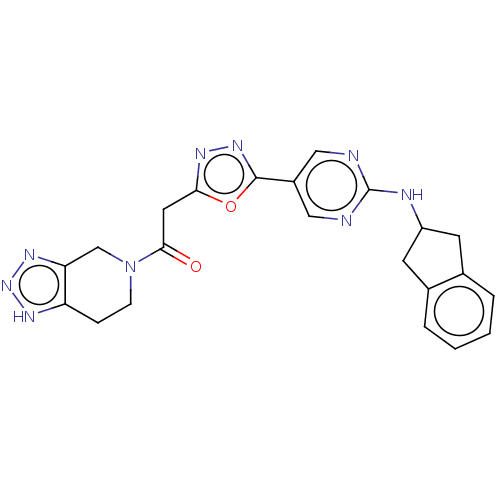 (Example 6-1 | US10961242, Compound 33 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489316 (Example 6-2 | US10961242, Compound 34 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 324 total ) | Next | Last >> |