 Found 1423 hits with Last Name = 'la regina' and Initial = 'g'
Found 1423 hits with Last Name = 'la regina' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133408
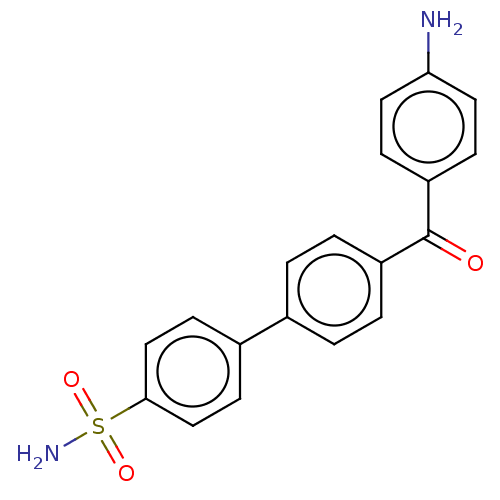
(CHEMBL3632844)Show SMILES Nc1ccc(cc1)C(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16N2O3S/c20-17-9-5-16(6-10-17)19(22)15-3-1-13(2-4-15)14-7-11-18(12-8-14)25(21,23)24/h1-12H,20H2,(H2,21,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133390
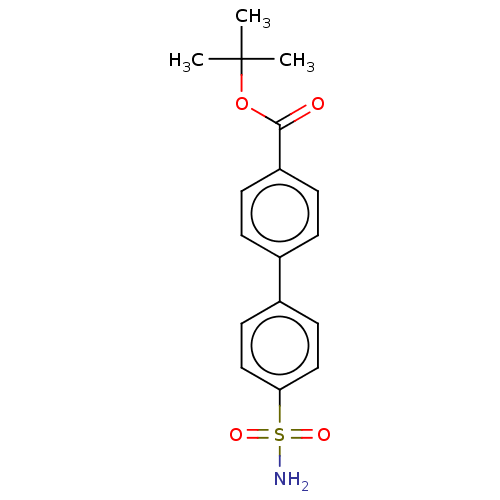
(CHEMBL3632826)Show SMILES CC(C)(C)OC(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H19NO4S/c1-17(2,3)22-16(19)14-6-4-12(5-7-14)13-8-10-15(11-9-13)23(18,20)21/h4-11H,1-3H3,(H2,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133394
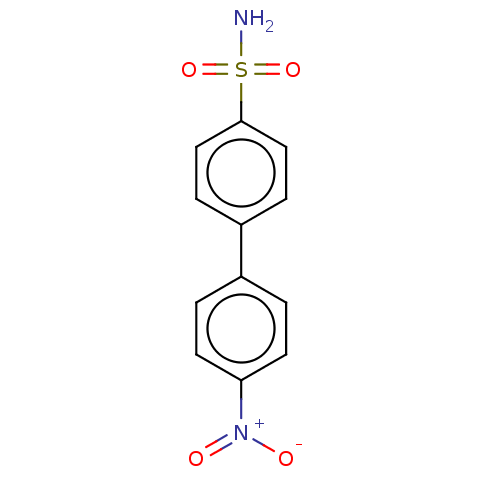
(CHEMBL3632830)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C12H10N2O4S/c13-19(17,18)12-7-3-10(4-8-12)9-1-5-11(6-2-9)14(15)16/h1-8H,(H2,13,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133400
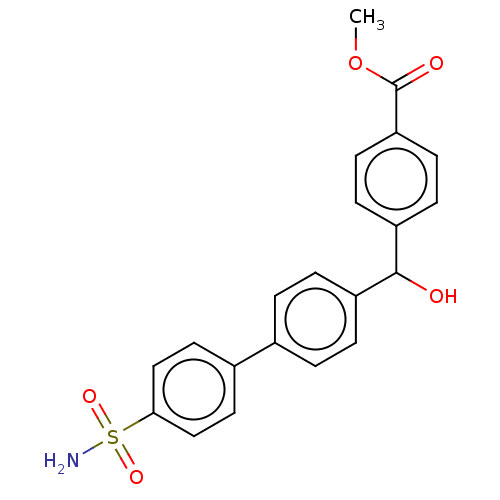
(CHEMBL3632836)Show SMILES COC(=O)c1ccc(cc1)C(O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H19NO5S/c1-27-21(24)18-8-6-17(7-9-18)20(23)16-4-2-14(3-5-16)15-10-12-19(13-11-15)28(22,25)26/h2-13,20,23H,1H3,(H2,22,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50356552
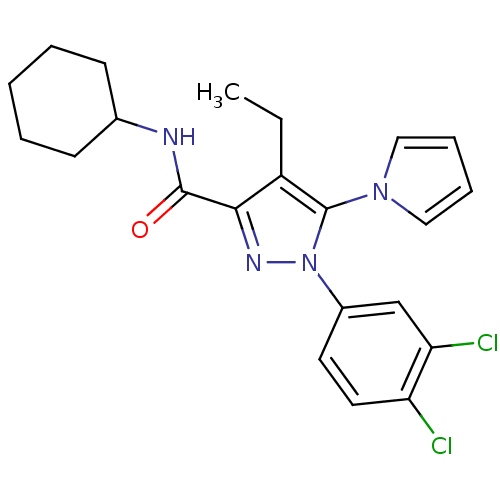
(CHEMBL1909860)Show SMILES CCc1c(nn(c1-n1cccc1)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H24Cl2N4O/c1-2-17-20(21(29)25-15-8-4-3-5-9-15)26-28(22(17)27-12-6-7-13-27)16-10-11-18(23)19(24)14-16/h6-7,10-15H,2-5,8-9H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540581

(CHEMBL4648640)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(Cc2cccc(c2)C(O)=O)cc1 Show InChI InChI=1S/C20H17NO4S/c21-26(24,25)19-10-8-17(9-11-19)16-6-4-14(5-7-16)12-15-2-1-3-18(13-15)20(22)23/h1-11,13H,12H2,(H,22,23)(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133389
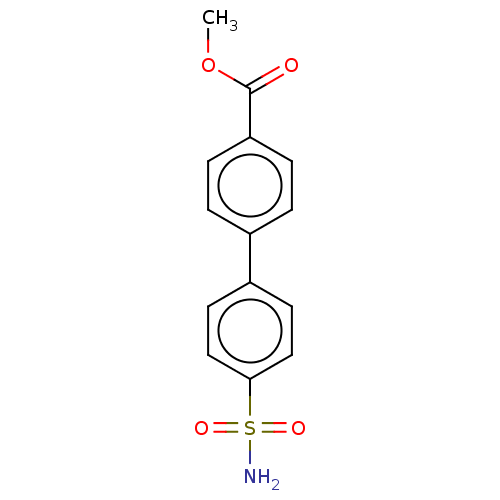
(CHEMBL3632825)Show InChI InChI=1S/C14H13NO4S/c1-19-14(16)12-4-2-10(3-5-12)11-6-8-13(9-7-11)20(15,17)18/h2-9H,1H3,(H2,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133409
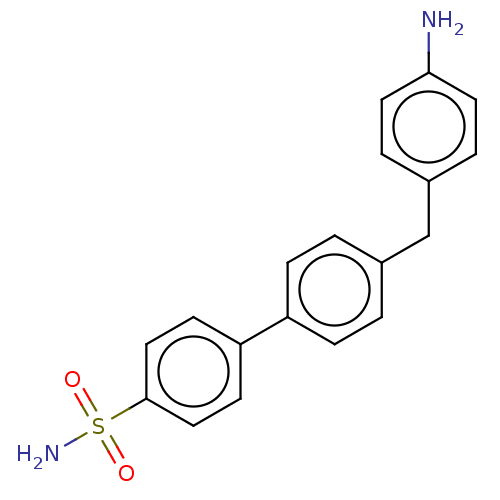
(CHEMBL3632845)Show SMILES Nc1ccc(Cc2ccc(cc2)-c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C19H18N2O2S/c20-18-9-3-15(4-10-18)13-14-1-5-16(6-2-14)17-7-11-19(12-8-17)24(21,22)23/h1-12H,13,20H2,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540579
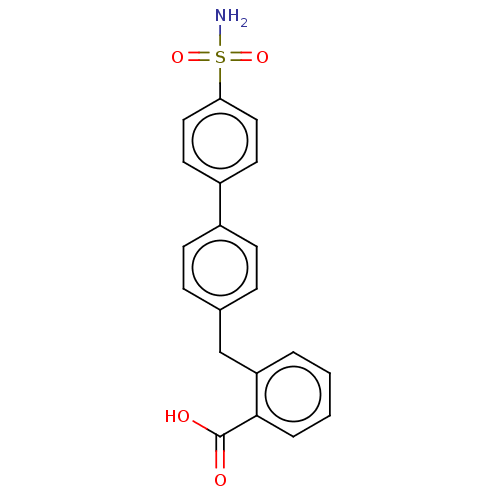
(CHEMBL4636586)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(Cc2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C20H17NO4S/c21-26(24,25)18-11-9-16(10-12-18)15-7-5-14(6-8-15)13-17-3-1-2-4-19(17)20(22)23/h1-12H,13H2,(H,22,23)(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133393

(CHEMBL3632829)Show InChI InChI=1S/C15H16N2O4S/c16-22(20,21)14-7-5-12(6-8-14)11-1-3-13(4-2-11)15(19)17-9-10-18/h1-8,18H,9-10H2,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540577

(CHEMBL4635511)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C1OC(=O)c2ccccc12 Show InChI InChI=1S/C20H15NO4S/c21-26(23,24)16-11-9-14(10-12-16)13-5-7-15(8-6-13)19-17-3-1-2-4-18(17)20(22)25-19/h1-12,19H,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133391
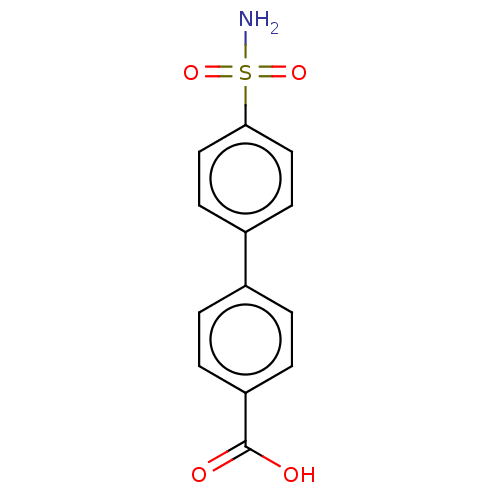
(CHEMBL3632827)Show InChI InChI=1S/C13H11NO4S/c14-19(17,18)12-7-5-10(6-8-12)9-1-3-11(4-2-9)13(15)16/h1-8H,(H,15,16)(H2,14,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133397
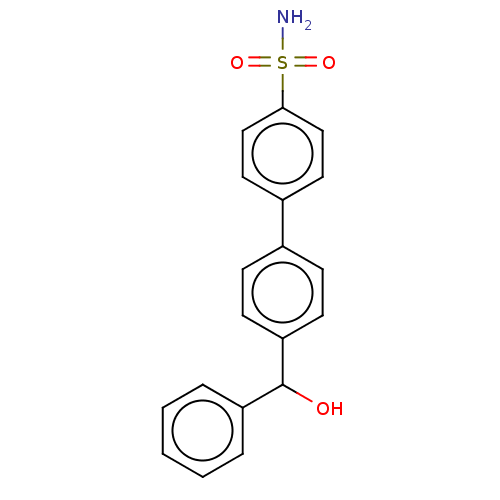
(CHEMBL3632833)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(O)c1ccccc1 Show InChI InChI=1S/C19H17NO3S/c20-24(22,23)18-12-10-15(11-13-18)14-6-8-17(9-7-14)19(21)16-4-2-1-3-5-16/h1-13,19,21H,(H2,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540576

(CHEMBL4633482)Show SMILES Nc1cccc(c1)C(O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H18N2O3S/c20-17-3-1-2-16(12-17)19(22)15-6-4-13(5-7-15)14-8-10-18(11-9-14)25(21,23)24/h1-12,19,22H,20H2,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540578

(CHEMBL4636187)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1ccccc1C(O)=O Show InChI InChI=1S/C20H15NO5S/c21-27(25,26)16-11-9-14(10-12-16)13-5-7-15(8-6-13)19(22)17-3-1-2-4-18(17)20(23)24/h1-12H,(H,23,24)(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133404

(CHEMBL3632840)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(Cc2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C20H17NO4S/c21-26(24,25)19-11-9-17(10-12-19)16-5-1-14(2-6-16)13-15-3-7-18(8-4-15)20(22)23/h1-12H,13H2,(H,22,23)(H2,21,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540582
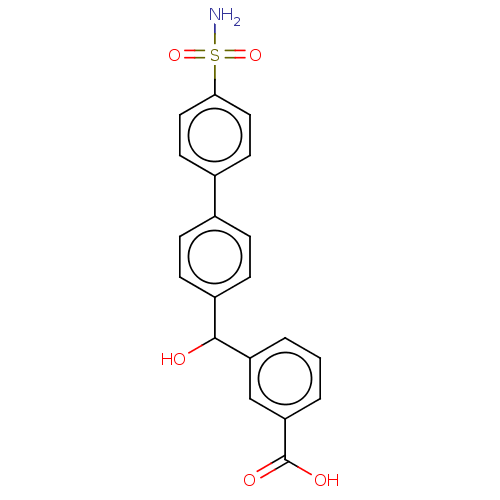
(CHEMBL4639123)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(O)c1cccc(c1)C(O)=O Show InChI InChI=1S/C20H17NO5S/c21-27(25,26)18-10-8-14(9-11-18)13-4-6-15(7-5-13)19(22)16-2-1-3-17(12-16)20(23)24/h1-12,19,22H,(H,23,24)(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133396
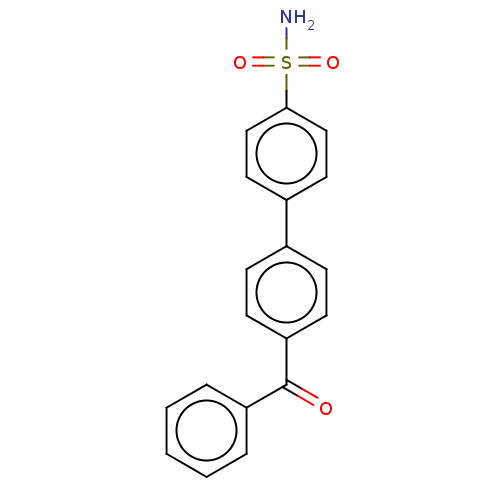
(CHEMBL3632832)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C19H15NO3S/c20-24(22,23)18-12-10-15(11-13-18)14-6-8-17(9-7-14)19(21)16-4-2-1-3-5-16/h1-13H,(H2,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50356548
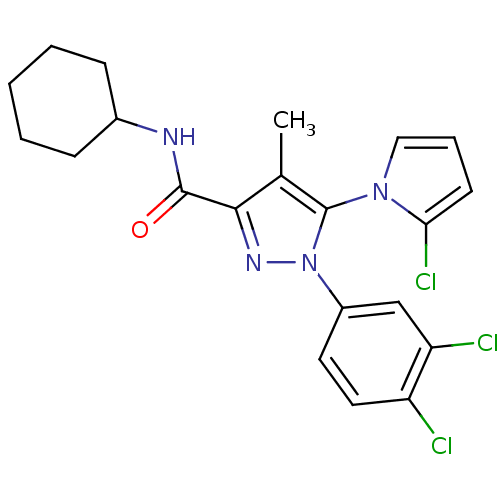
(CHEMBL1909856)Show SMILES Cc1c(nn(c1-n1cccc1Cl)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 |(9.73,-1.52,;10.63,-2.77,;12.17,-2.77,;12.64,-4.23,;11.4,-5.13,;10.15,-4.23,;8.68,-4.68,;8.18,-6.13,;6.64,-6.11,;6.19,-4.64,;7.45,-3.75,;7.47,-2.21,;11.39,-6.67,;10.05,-7.44,;10.05,-8.97,;11.38,-9.75,;11.38,-11.29,;12.72,-8.97,;14.06,-9.74,;12.72,-7.43,;13.07,-1.52,;12.45,-.12,;14.61,-1.69,;15.51,-.44,;17.05,-.61,;17.96,.62,;17.34,2.03,;15.81,2.2,;14.89,.96,)| Show InChI InChI=1S/C21H21Cl3N4O/c1-13-19(20(29)25-14-6-3-2-4-7-14)26-28(15-9-10-16(22)17(23)12-15)21(13)27-11-5-8-18(27)24/h5,8-12,14H,2-4,6-7H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50273531

(CHEMBL460599 | N-Methyl,N-(3-phenylpropyl)-1H-indo...)Show InChI InChI=1S/C19H20N2O/c1-21(13-7-10-15-8-3-2-4-9-15)19(22)18-14-16-11-5-6-12-17(16)20-18/h2-6,8-9,11-12,14,20H,7,10,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial MAO-A by fluorometric assay |
Bioorg Med Chem 16: 9729-40 (2008)
Article DOI: 10.1016/j.bmc.2008.09.072
BindingDB Entry DOI: 10.7270/Q20001ZK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540574

(CHEMBL4636115)Show SMILES Nc1cccc(Cc2ccc(cc2)-c2ccc(cc2)S(N)(=O)=O)c1 Show InChI InChI=1S/C19H18N2O2S/c20-18-3-1-2-15(13-18)12-14-4-6-16(7-5-14)17-8-10-19(11-9-17)24(21,22)23/h1-11,13H,12,20H2,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133402
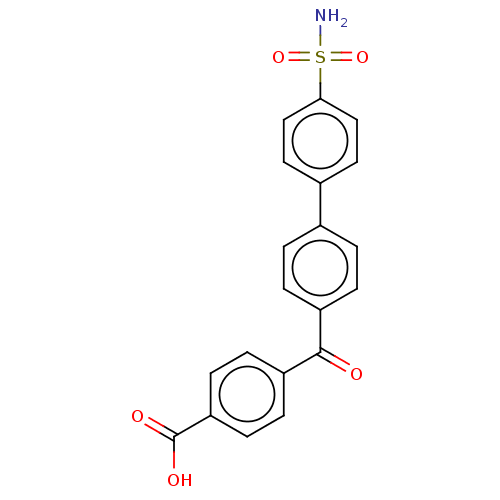
(CHEMBL3632838)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H15NO5S/c21-27(25,26)18-11-9-14(10-12-18)13-1-3-15(4-2-13)19(22)16-5-7-17(8-6-16)20(23)24/h1-12H,(H,23,24)(H2,21,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540580

(CHEMBL4642918)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1cccc(c1)C(O)=O Show InChI InChI=1S/C20H15NO5S/c21-27(25,26)18-10-8-14(9-11-18)13-4-6-15(7-5-13)19(22)16-2-1-3-17(12-16)20(23)24/h1-12H,(H,23,24)(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540575
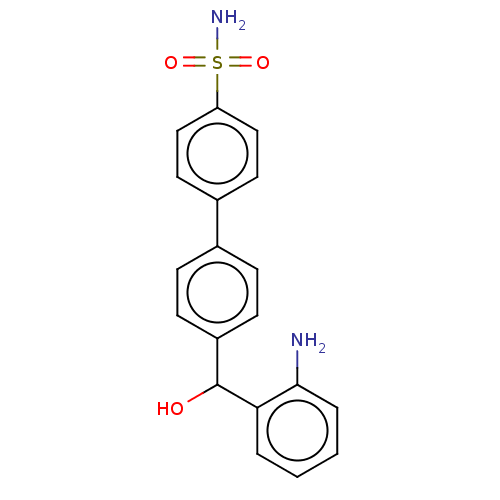
(CHEMBL4647509)Show SMILES Nc1ccccc1C(O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H18N2O3S/c20-18-4-2-1-3-17(18)19(22)15-7-5-13(6-8-15)14-9-11-16(12-10-14)25(21,23)24/h1-12,19,22H,20H2,(H2,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 10 mins by phenol red dye based stopped flow CO2 hydration assay |
ACS Med Chem Lett 11: 633-637 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00437
BindingDB Entry DOI: 10.7270/Q23J3HH4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50273595

(CHEMBL462097 | N-(1H-Indol-2-ylmethyl)-N-methyl-N-...)Show InChI InChI=1S/C18H20N2/c1-20(12-11-15-7-3-2-4-8-15)14-17-13-16-9-5-6-10-18(16)19-17/h2-10,13,19H,11-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial MAO-A by fluorometric assay |
Bioorg Med Chem 16: 9729-40 (2008)
Article DOI: 10.1016/j.bmc.2008.09.072
BindingDB Entry DOI: 10.7270/Q20001ZK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21266
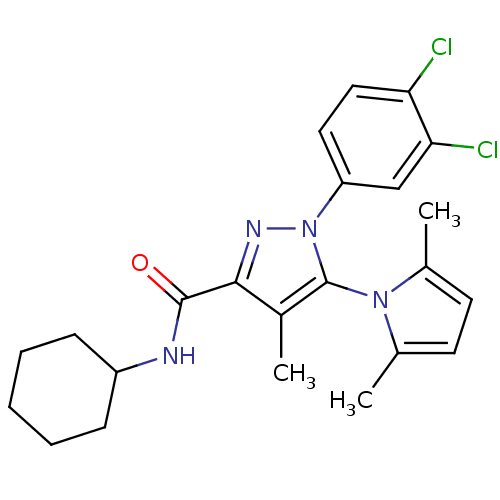
(N-cyclohexyl-1-(3,4-dichlorophenyl)-5-(2,5-dimethy...)Show SMILES Cc1ccc(C)n1-c1c(C)c(nn1-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 |(-5.72,5.81,;-5.72,4.27,;-6.96,3.36,;-6.49,1.9,;-4.95,1.9,;-4.18,.56,;-4.47,3.36,;-3.02,3.89,;-2.6,5.36,;-3.54,6.58,;-1.06,5.42,;-.53,3.97,;-1.75,3.02,;-1.75,1.48,;-3.08,.71,;-3.08,-.83,;-1.75,-1.6,;-1.75,-3.14,;-.42,-.83,;.92,-1.6,;-.42,.71,;-.19,6.69,;-.87,8.08,;1.34,6.58,;2.68,7.35,;3.95,6.49,;5.34,7.17,;5.44,8.7,;4.16,9.56,;2.78,8.89,)| Show InChI InChI=1S/C23H26Cl2N4O/c1-14-9-10-15(2)28(14)23-16(3)21(22(30)26-17-7-5-4-6-8-17)27-29(23)18-11-12-19(24)20(25)13-18/h9-13,17H,4-8H2,1-3H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 51: 1560-76 (2008)
Article DOI: 10.1021/jm070566z
BindingDB Entry DOI: 10.7270/Q2GX48V9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50356550
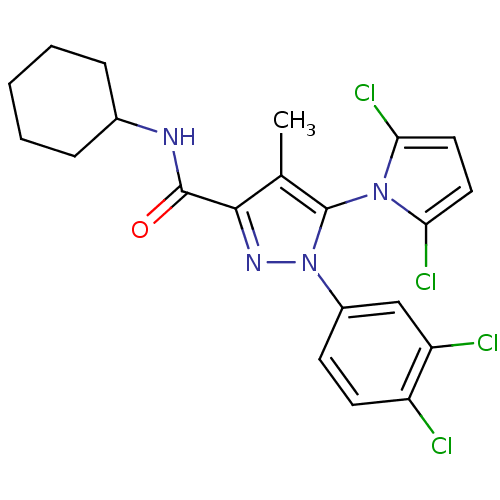
(CHEMBL1909858)Show SMILES Cc1c(nn(c1-n1c(Cl)ccc1Cl)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 |(33.16,-36.05,;34.06,-37.29,;35.6,-37.3,;36.07,-38.76,;34.82,-39.66,;33.58,-38.75,;32.11,-39.21,;30.88,-38.28,;30.9,-36.74,;29.62,-39.16,;30.07,-40.64,;31.61,-40.66,;32.5,-41.92,;34.82,-41.2,;33.48,-41.96,;33.48,-43.5,;34.81,-44.27,;34.81,-45.81,;36.15,-43.5,;37.49,-44.27,;36.15,-41.96,;36.5,-36.05,;35.88,-34.64,;38.03,-36.21,;38.94,-34.97,;40.47,-35.14,;41.38,-33.9,;40.76,-32.49,;39.22,-32.32,;38.31,-33.57,)| Show InChI InChI=1S/C21H20Cl4N4O/c1-12-19(20(30)26-13-5-3-2-4-6-13)27-29(14-7-8-15(22)16(23)11-14)21(12)28-17(24)9-10-18(28)25/h7-11,13H,2-6H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50298944
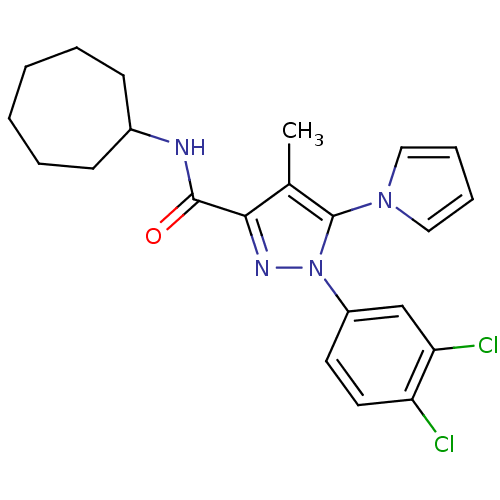
(CHEMBL576549 | N-Cycloheptyl-1-(3,4-dichlorophenyl...)Show SMILES Cc1c(nn(c1-n1cccc1)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C22H24Cl2N4O/c1-15-20(21(29)25-16-8-4-2-3-5-9-16)26-28(22(15)27-12-6-7-13-27)17-10-11-18(23)19(24)14-17/h6-7,10-14,16H,2-5,8-9H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50273561
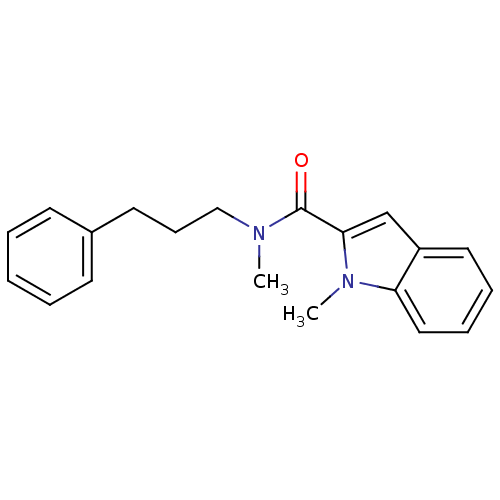
(CHEMBL459336 | N-Methyl,N-(3-phenylpropyl)-1-methy...)Show InChI InChI=1S/C20H22N2O/c1-21(14-8-11-16-9-4-3-5-10-16)20(23)19-15-17-12-6-7-13-18(17)22(19)2/h3-7,9-10,12-13,15H,8,11,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial MAO-B by fluorometric assay |
Bioorg Med Chem 16: 9729-40 (2008)
Article DOI: 10.1016/j.bmc.2008.09.072
BindingDB Entry DOI: 10.7270/Q20001ZK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50298942
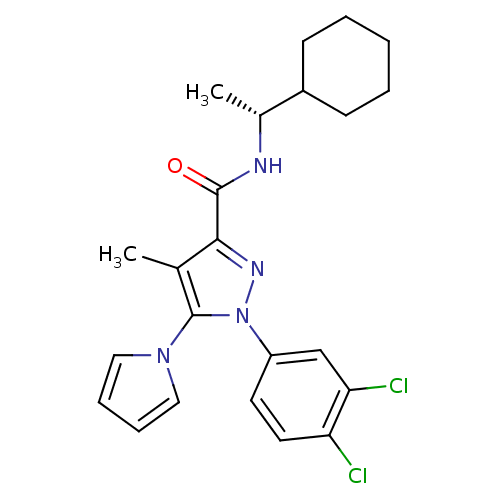
((R)-N-[1-(1-Cyclohexyl)ethyl]1-(3,4-dichlorophenyl...)Show SMILES C[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)c(Cl)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H26Cl2N4O/c1-15-21(22(30)26-16(2)17-8-4-3-5-9-17)27-29(23(15)28-12-6-7-13-28)18-10-11-19(24)20(25)14-18/h6-7,10-14,16-17H,3-5,8-9H2,1-2H3,(H,26,30)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21263
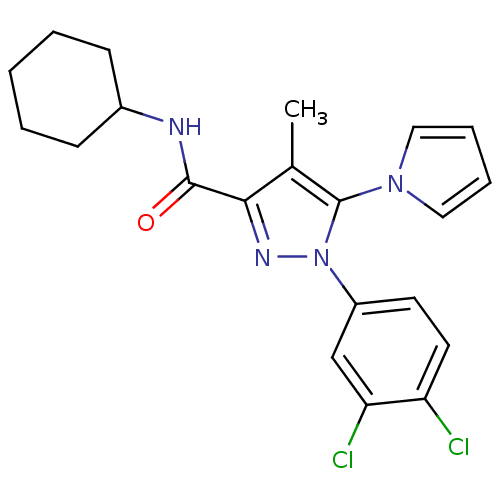
(N-cyclohexyl-1-(3,4-dichlorophenyl)-4-methyl-5-(1H...)Show SMILES Cc1c(nn(c1-n1cccc1)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C21H22Cl2N4O/c1-14-19(20(28)24-15-7-3-2-4-8-15)25-27(21(14)26-11-5-6-12-26)16-9-10-17(22)18(23)13-16/h5-6,9-13,15H,2-4,7-8H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 51: 1560-76 (2008)
Article DOI: 10.1021/jm070566z
BindingDB Entry DOI: 10.7270/Q2GX48V9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50356554
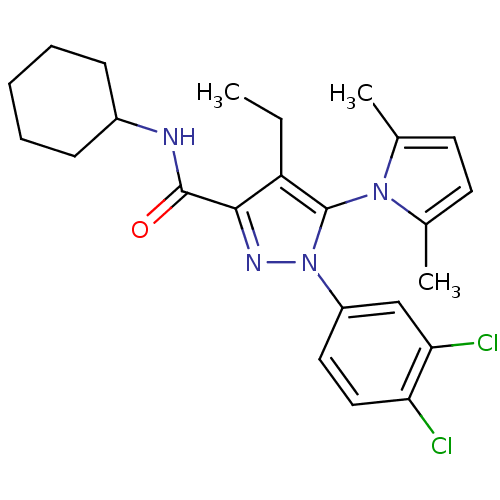
(CHEMBL1909957)Show SMILES CCc1c(nn(c1-n1c(C)ccc1C)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 |(34.91,5.92,;34.28,4.51,;35.19,3.27,;36.72,3.27,;37.2,1.8,;35.95,.9,;34.71,1.81,;33.24,1.35,;32,2.28,;32.03,3.82,;30.75,1.4,;31.2,-.08,;32.74,-.1,;33.22,-1.53,;35.95,-.63,;34.61,-1.4,;34.61,-2.94,;35.94,-3.71,;35.94,-5.25,;37.28,-2.94,;38.62,-3.7,;37.28,-1.4,;37.63,4.51,;37.01,5.92,;39.16,4.35,;40.07,5.59,;41.6,5.42,;42.5,6.66,;41.89,8.08,;40.35,8.25,;39.43,6.99,)| Show InChI InChI=1S/C24H28Cl2N4O/c1-4-19-22(23(31)27-17-8-6-5-7-9-17)28-30(18-12-13-20(25)21(26)14-18)24(19)29-15(2)10-11-16(29)3/h10-14,17H,4-9H2,1-3H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21263

(N-cyclohexyl-1-(3,4-dichlorophenyl)-4-methyl-5-(1H...)Show SMILES Cc1c(nn(c1-n1cccc1)-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C21H22Cl2N4O/c1-14-19(20(28)24-15-7-3-2-4-8-15)25-27(21(14)26-11-5-6-12-26)16-9-10-17(22)18(23)13-16/h5-6,9-13,15H,2-4,7-8H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM15604
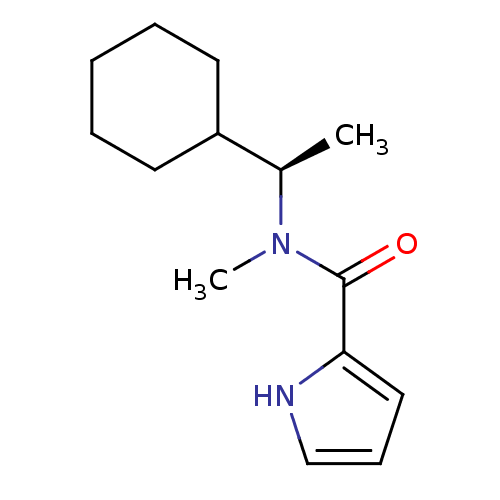
((R)-N-(alpha-Cyclohexylethyl),N-methyl-1H-pyrrole-...)Show InChI InChI=1S/C14H22N2O/c1-11(12-7-4-3-5-8-12)16(2)14(17)13-9-6-10-15-13/h6,9-12,15H,3-5,7-8H2,1-2H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 50: 922-31 (2007)
Article DOI: 10.1021/jm060882y
BindingDB Entry DOI: 10.7270/Q2GH9G61 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279
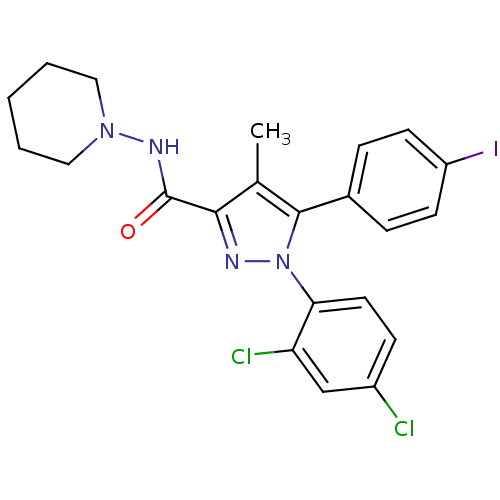
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universitāadi Roma
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 51: 1560-76 (2008)
Article DOI: 10.1021/jm070566z
BindingDB Entry DOI: 10.7270/Q2GX48V9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279

(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB1 receptor transfected in HEK cells |
Eur J Med Chem 45: 5878-86 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.053
BindingDB Entry DOI: 10.7270/Q22Z15T3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279

(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50356540
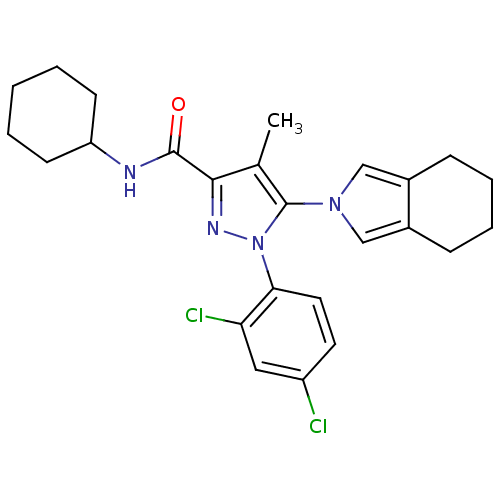
(CHEMBL1909848)Show SMILES Cc1c(nn(c1-n1cc2CCCCc2c1)-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C25H28Cl2N4O/c1-16-23(24(32)28-20-9-3-2-4-10-20)29-31(22-12-11-19(26)13-21(22)27)25(16)30-14-17-7-5-6-8-18(17)15-30/h11-15,20H,2-10H2,1H3,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB1 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50298941
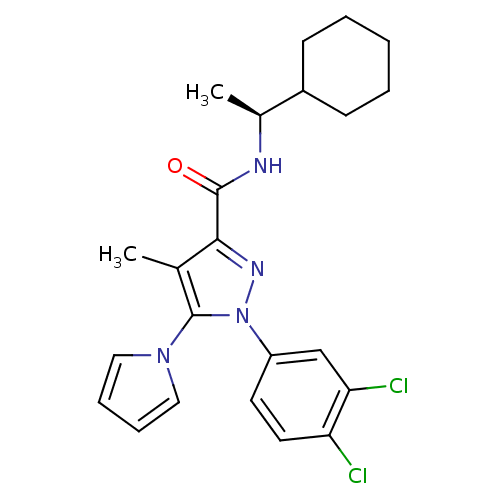
((S)-N-[1-(1-Cyclohexyl)ethyl]1-(3,4-dichlorophenyl...)Show SMILES C[C@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)c(Cl)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H26Cl2N4O/c1-15-21(22(30)26-16(2)17-8-4-3-5-9-17)27-29(23(15)28-12-6-7-13-28)18-10-11-19(24)20(25)14-18/h6-7,10-14,16-17H,3-5,8-9H2,1-2H3,(H,26,30)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50356542
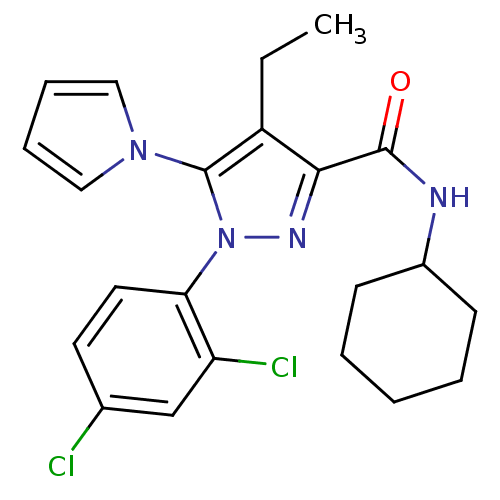
(CHEMBL1909850)Show SMILES CCc1c(nn(c1-n1cccc1)-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H24Cl2N4O/c1-2-17-20(21(29)25-16-8-4-3-5-9-16)26-28(22(17)27-12-6-7-13-27)19-11-10-15(23)14-18(19)24/h6-7,10-14,16H,2-5,8-9H2,1H3,(H,25,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from recombinant human CB1 receptor expressed in HEK cells |
Eur J Med Chem 46: 5641-53 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.037
BindingDB Entry DOI: 10.7270/Q2P55NXD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50298941

((S)-N-[1-(1-Cyclohexyl)ethyl]1-(3,4-dichlorophenyl...)Show SMILES C[C@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)c(Cl)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H26Cl2N4O/c1-15-21(22(30)26-16(2)17-8-4-3-5-9-17)27-29(23(15)28-12-6-7-13-28)18-10-11-19(24)20(25)14-18/h6-7,10-14,16-17H,3-5,8-9H2,1-2H3,(H,26,30)/t16-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50273486

(CHEMBL464597 | N-Methyl,N-phenyl-1H-indole-2-carbo...)Show InChI InChI=1S/C16H14N2O/c1-18(13-8-3-2-4-9-13)16(19)15-11-12-7-5-6-10-14(12)17-15/h2-11,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial MAO-A by fluorometric assay |
Bioorg Med Chem 16: 9729-40 (2008)
Article DOI: 10.1016/j.bmc.2008.09.072
BindingDB Entry DOI: 10.7270/Q20001ZK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50133391

(CHEMBL3632827)Show InChI InChI=1S/C13H11NO4S/c14-19(17,18)12-7-5-10(6-8-12)9-1-3-11(4-2-9)13(15)16/h1-8H,(H,15,16)(H2,14,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-9 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133405
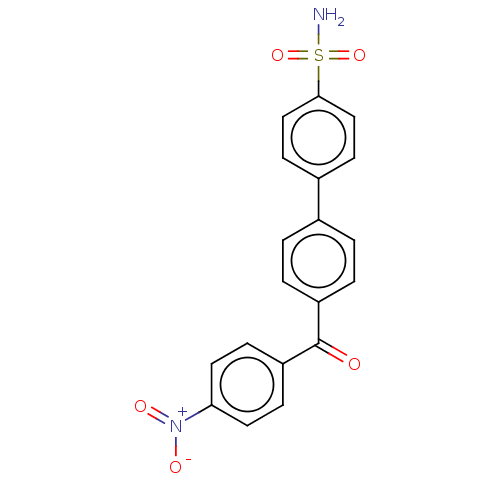
(CHEMBL3632841)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H14N2O5S/c20-27(25,26)18-11-7-14(8-12-18)13-1-3-15(4-2-13)19(22)16-5-9-17(10-6-16)21(23)24/h1-12H,(H2,20,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133395
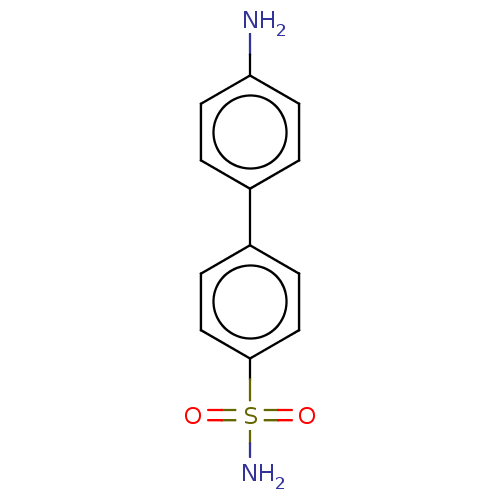
(CHEMBL3632831)Show InChI InChI=1S/C12H12N2O2S/c13-11-5-1-9(2-6-11)10-3-7-12(8-4-10)17(14,15)16/h1-8H,13H2,(H2,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133407
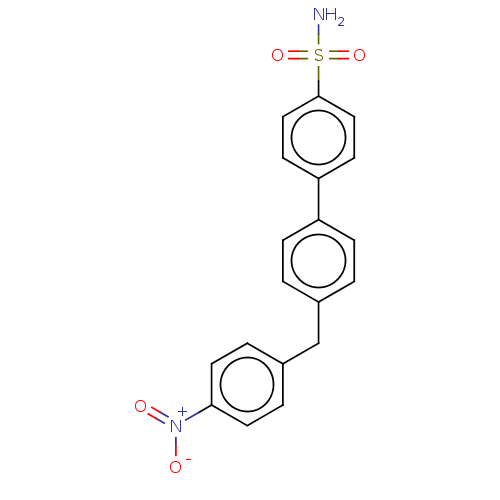
(CHEMBL3632843)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(Cc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C19H16N2O4S/c20-26(24,25)19-11-7-17(8-12-19)16-5-1-14(2-6-16)13-15-3-9-18(10-4-15)21(22)23/h1-12H,13H2,(H2,20,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
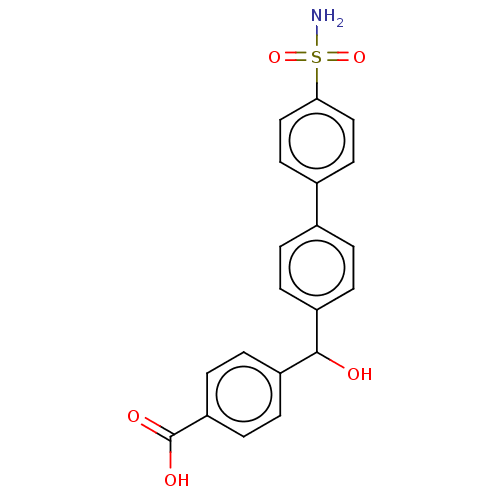
(Homo sapiens (Human)) | BDBM50133403
(CHEMBL3632839)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H17NO5S/c21-27(25,26)18-11-9-14(10-12-18)13-1-3-15(4-2-13)19(22)16-5-7-17(8-6-16)20(23)24/h1-12,19,22H,(H,23,24)(H2,21,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50273528
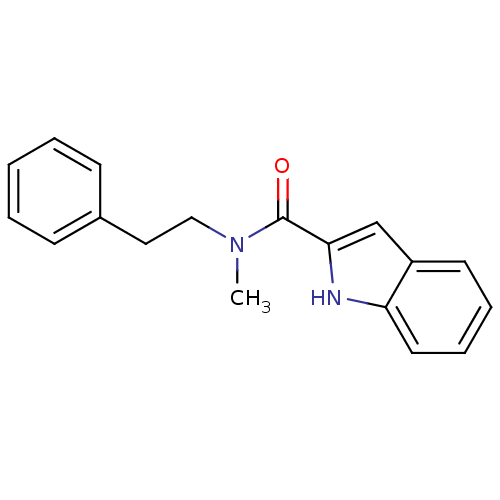
(CHEMBL460608 | N-Methyl,N-(2-phenylethyl)-1H-indol...)Show InChI InChI=1S/C18H18N2O/c1-20(12-11-14-7-3-2-4-8-14)18(21)17-13-15-9-5-6-10-16(15)19-17/h2-10,13,19H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondrial MAO-A by fluorometric assay |
Bioorg Med Chem 16: 9729-40 (2008)
Article DOI: 10.1016/j.bmc.2008.09.072
BindingDB Entry DOI: 10.7270/Q20001ZK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50298936
(CHEMBL579249 | N-Cycloheptyl-1-(3,4-dichlorophenyl...)Show SMILES Cc1ccc(C)n1-c1c(C)c(nn1-c1ccc(Cl)c(Cl)c1)C(=O)NC1CCCCCC1 |(31.5,-24.15,;32.38,-22.88,;33.91,-22.86,;34.37,-21.39,;33.1,-20.5,;33.07,-18.96,;31.88,-21.43,;30.4,-20.98,;29.9,-19.53,;30.78,-18.26,;28.36,-19.56,;27.91,-21.02,;29.17,-21.91,;29.2,-23.45,;27.88,-24.24,;27.91,-25.78,;29.26,-26.52,;29.29,-28.06,;30.58,-25.73,;31.92,-26.48,;30.55,-24.2,;27.44,-18.33,;28.03,-16.91,;25.91,-18.52,;24.96,-17.28,;23.48,-17.59,;22.3,-16.6,;22.34,-15.06,;23.56,-14.14,;25.05,-14.51,;25.71,-15.94,)| Show InChI InChI=1S/C24H28Cl2N4O/c1-15-10-11-16(2)29(15)24-17(3)22(23(31)27-18-8-6-4-5-7-9-18)28-30(24)19-12-13-20(25)21(26)14-19/h10-14,18H,4-9H2,1-3H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 17: 5549-64 (2009)
Article DOI: 10.1016/j.bmc.2009.06.027
BindingDB Entry DOI: 10.7270/Q2XK8GHD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50133396

(CHEMBL3632832)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C19H15NO3S/c20-24(22,23)18-12-10-15(11-13-18)14-6-8-17(9-7-14)19(21)16-4-2-1-3-5-16/h1-13H,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data