

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50465935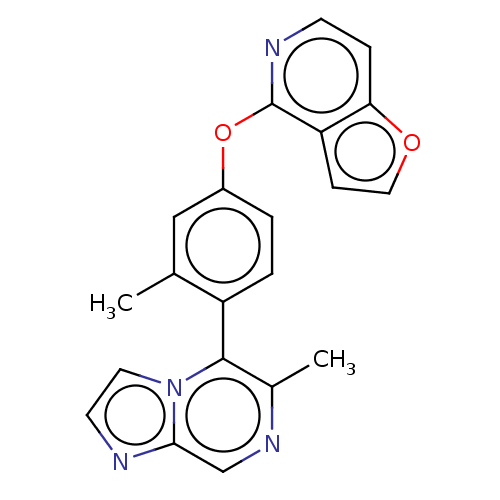 (CHEMBL4277264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at dopamine D5 receptor (unknown origin) | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465945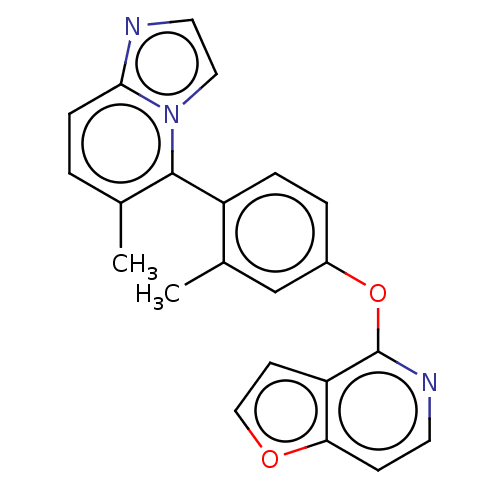 (CHEMBL4279267) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465944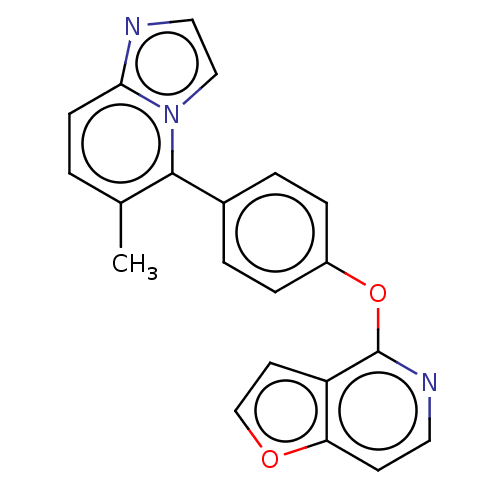 (CHEMBL4286177) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465946 (CHEMBL4294397) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465943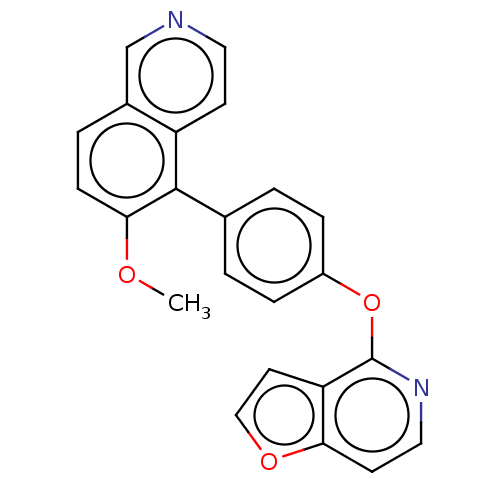 (CHEMBL4286110) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465938 (CHEMBL4294009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465934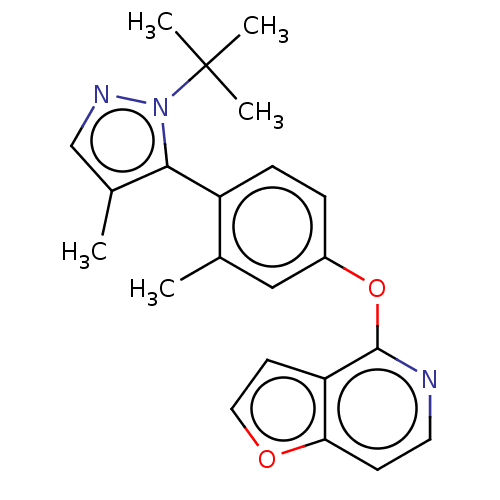 (CHEMBL4293757) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM55121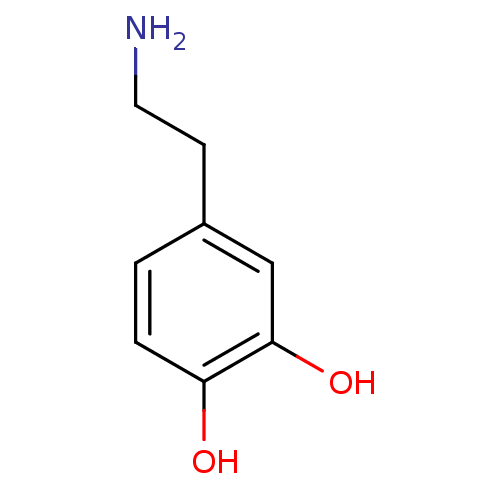 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465942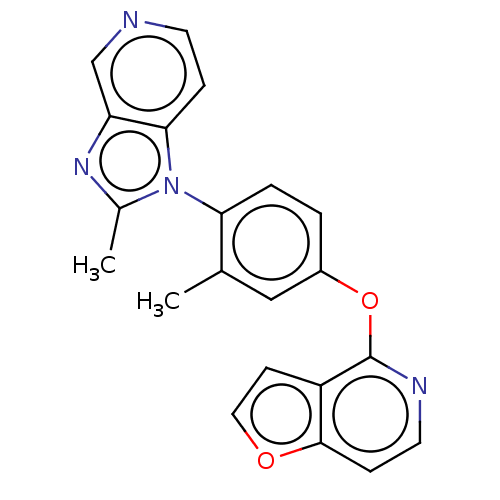 (CHEMBL4278861) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465936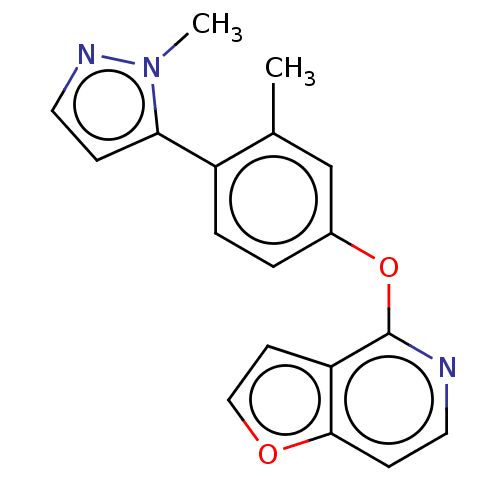 (CHEMBL4293356) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465947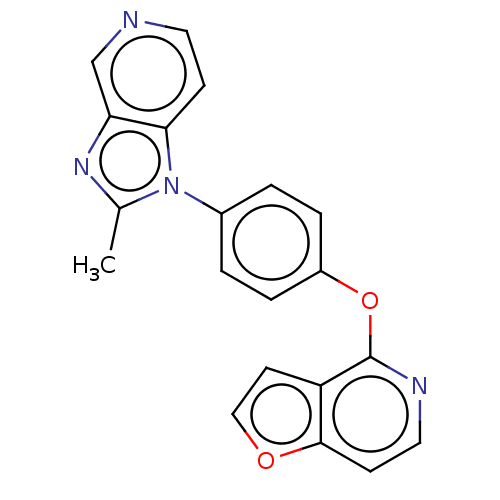 (CHEMBL4289538) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465941 (CHEMBL4283176) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465939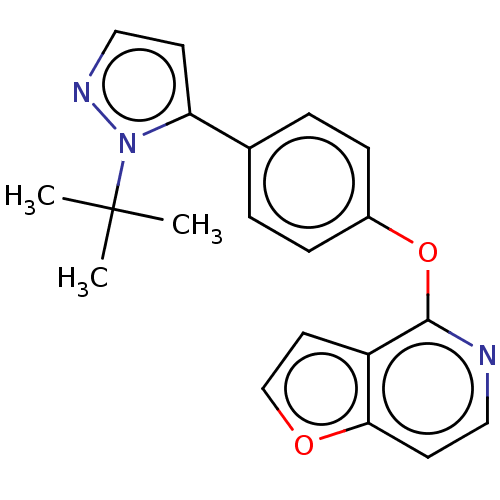 (CHEMBL4285528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465940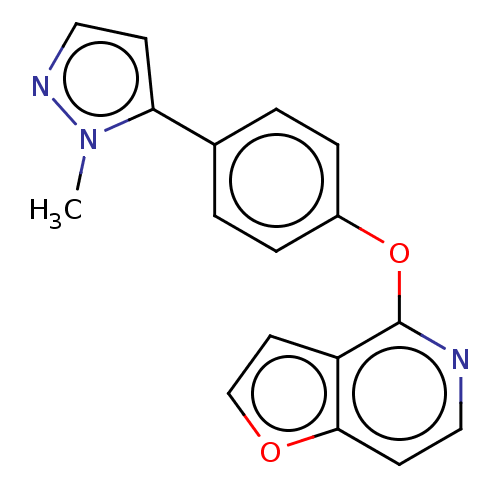 (CHEMBL4282096) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465937 (CHEMBL4287192) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332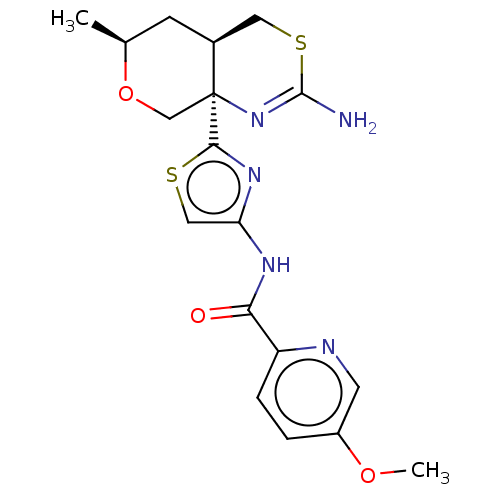 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875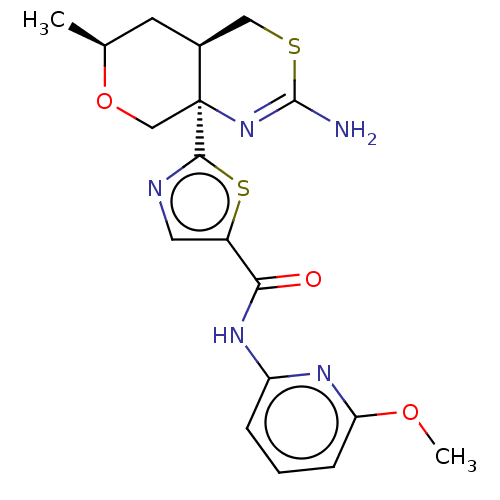 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884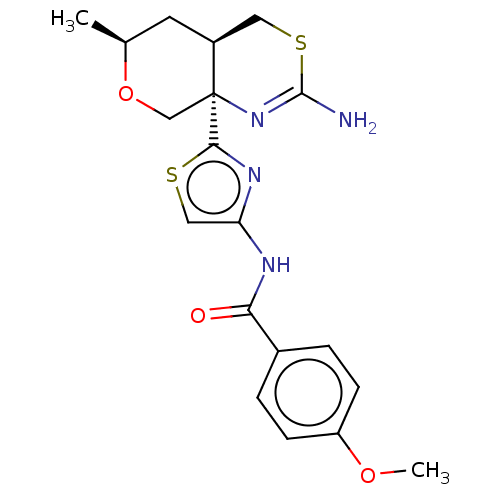 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577172 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-3,4,7,8...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353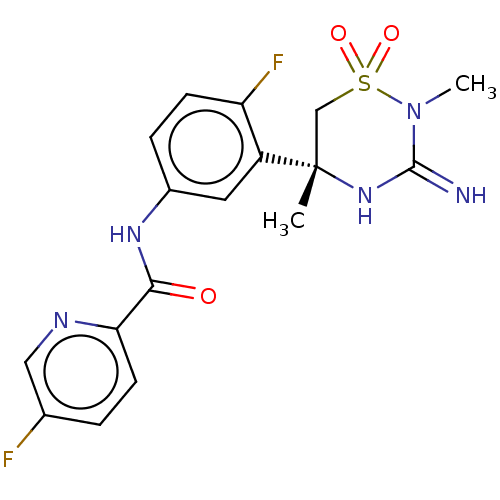 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577135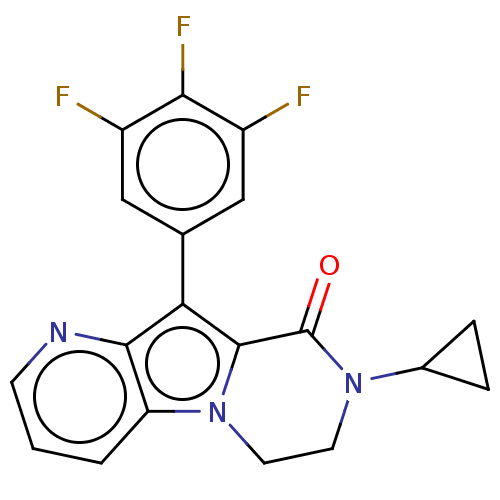 (8-Cyclopropyl-10-(3,4,5-trifluorophenyl)-7,8-dihyd...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577077 (10-(4-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577126 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285634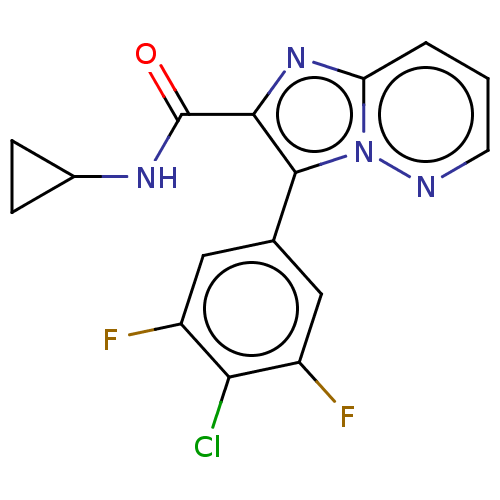 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (RD1) (Homo sapiens (Human)) | BDBM285634 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285634 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218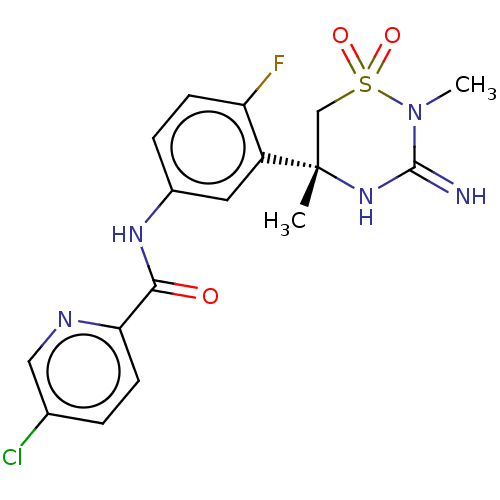 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577172 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-3,4,7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C [2-712] () | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874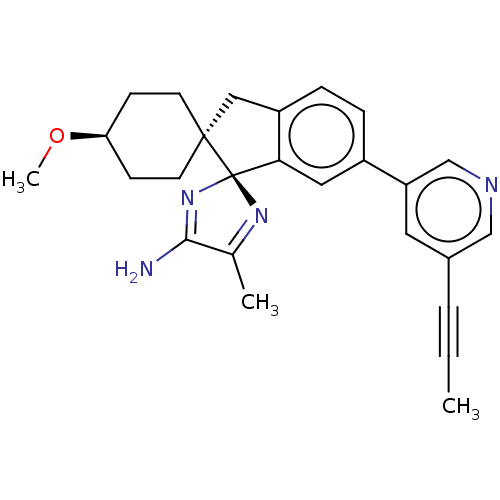 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577128 (2-Chloro-5-(8-cyclopropyl-9-oxo-6,7,8,9-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285598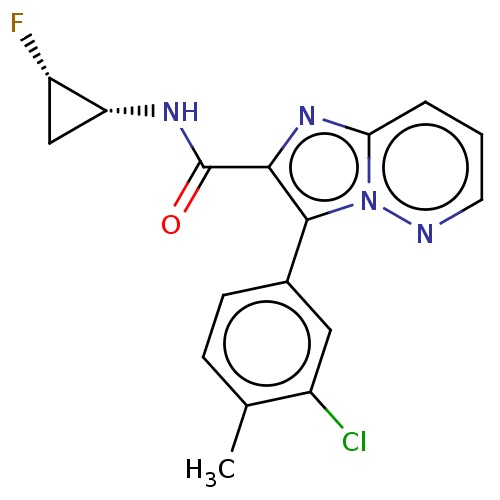 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (RD1) (Homo sapiens (Human)) | BDBM285598 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285598 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577135 (8-Cyclopropyl-10-(3,4,5-trifluorophenyl)-7,8-dihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577130 (10-(3-Chloro-4-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312935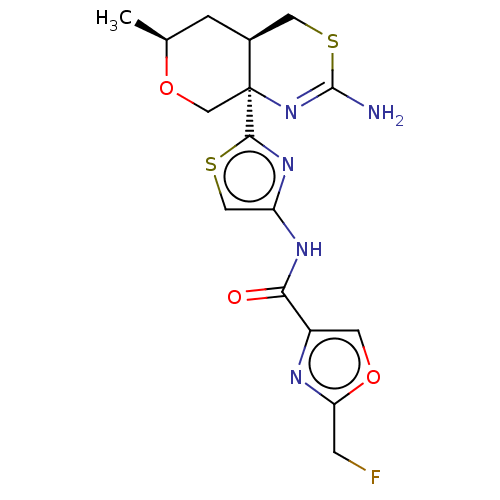 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Di... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577126 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577143 (10-(3-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577079 (4-(8-Cyclopropyl-9-oxo-6,7,8,9-tetrahydropyrido[2&...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577173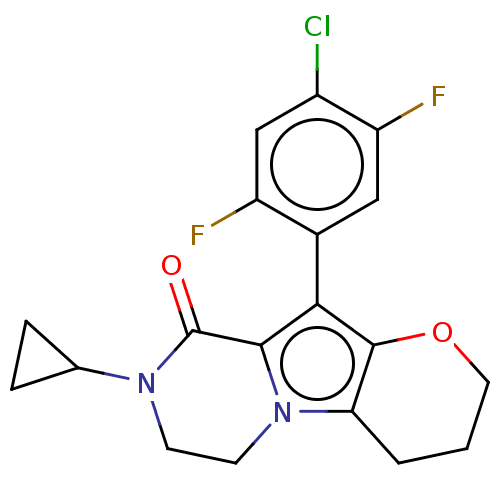 (10-(4-Chloro-2,5-difluorophenyl)-8-cyclopropyl-3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2788 total ) | Next | Last >> |