

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (IBV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666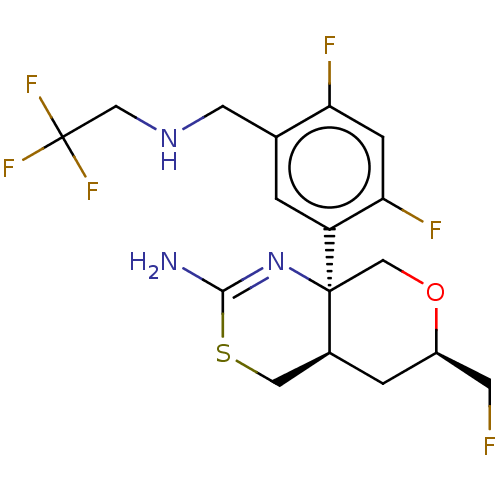 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332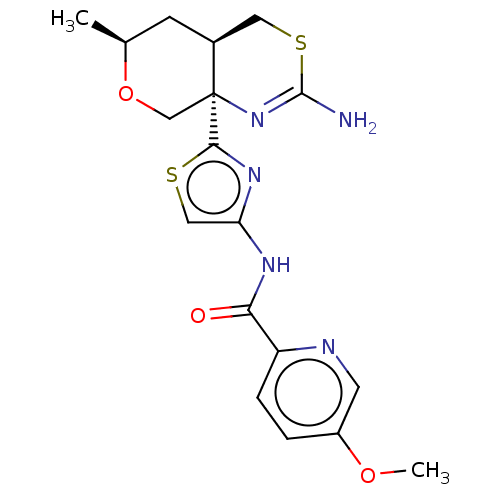 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875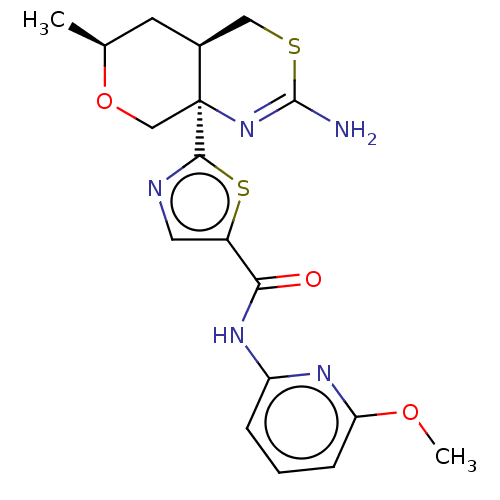 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884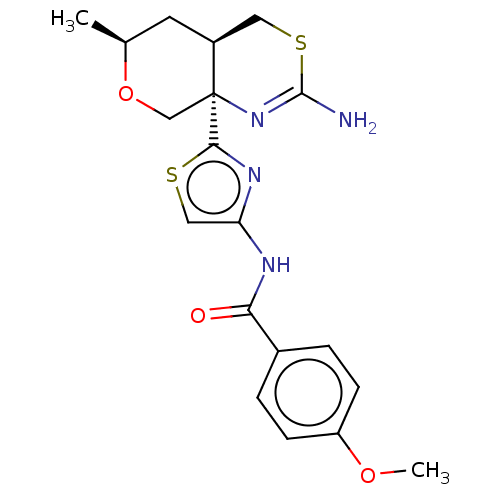 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353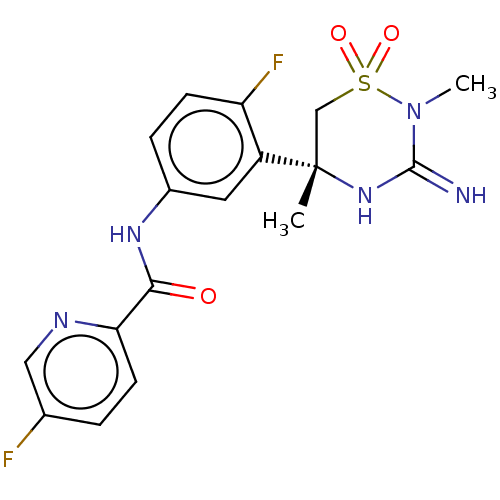 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218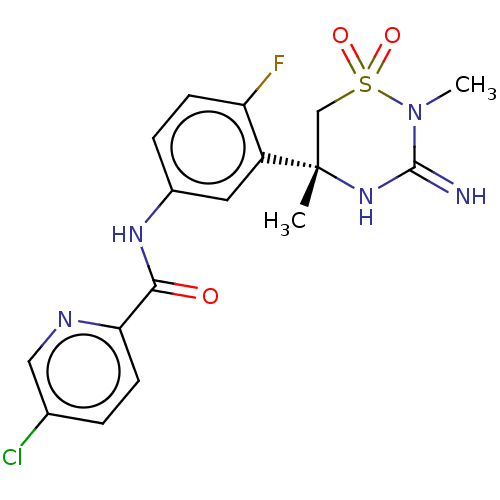 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874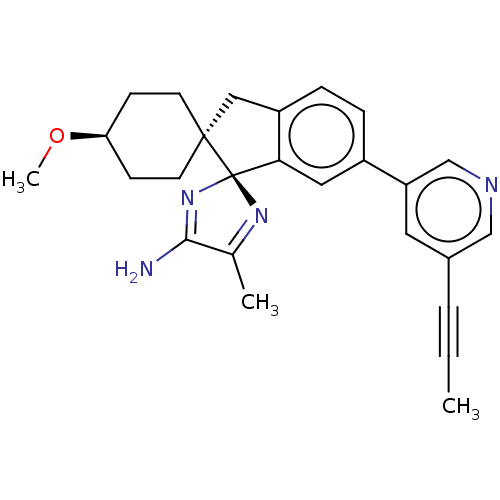 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452874 (CHEMBL4217620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259964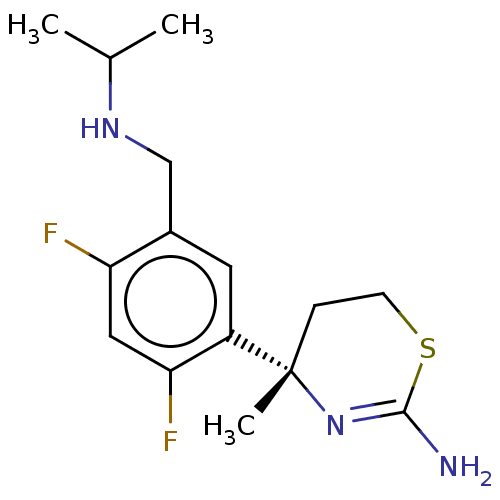 (CHEMBL4083698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223400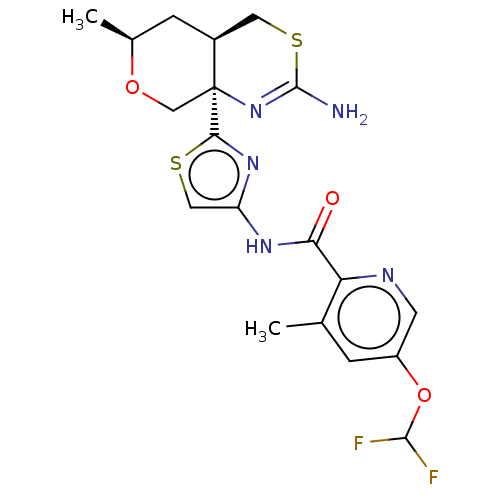 (US9315520, 45 | US9315520, Example 45 | US9605007,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length Myc-DDK-tagged BACE2 expressed in HEK293 cell membranes after 30 mins by parallel ... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223396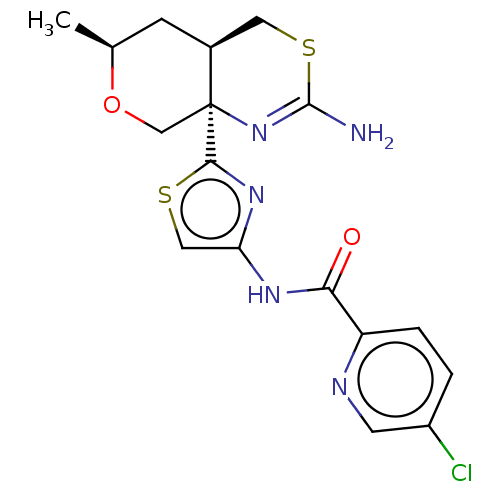 (US9315520, 7 | US9315520, Example 7 | US9605007, E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312940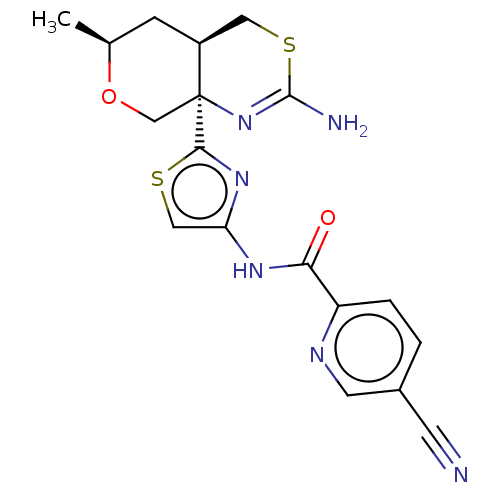 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259974 (CHEMBL4102593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259968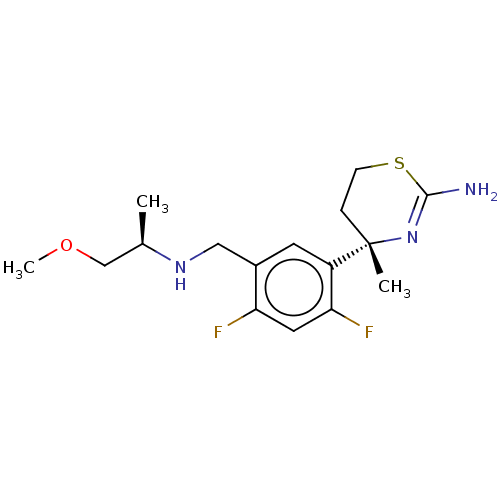 (CHEMBL4084653) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223330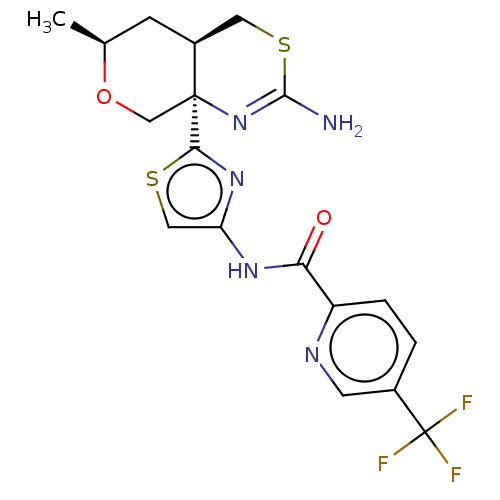 (US9315520, 17 | US9605007, Example 17 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395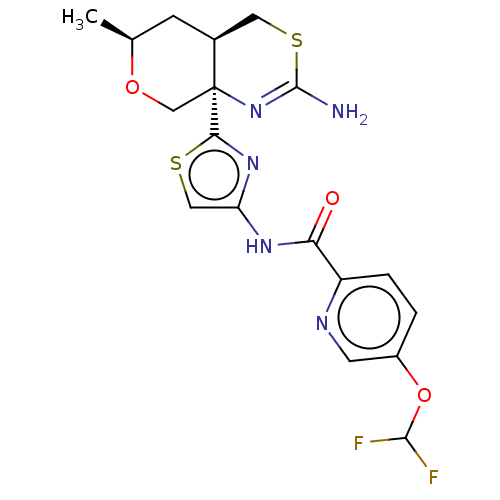 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259966 (CHEMBL4092406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50337557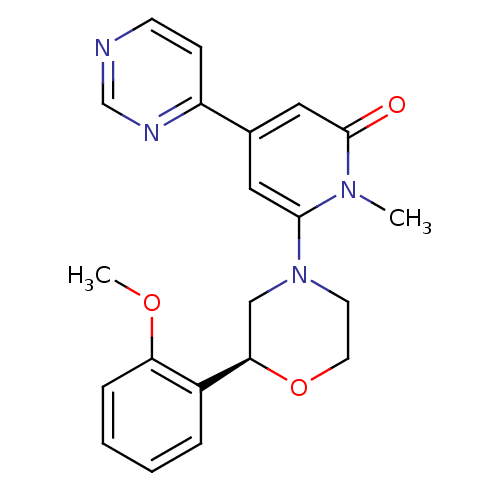 ((S)-6-(2-(2-methoxyphenyl)morpholino)-1-methyl-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta expressed in baculovirus infected SF9 cells after 60 mins | Bioorg Med Chem Lett 21: 1429-33 (2011) Article DOI: 10.1016/j.bmcl.2011.01.017 BindingDB Entry DOI: 10.7270/Q2KH0NNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312934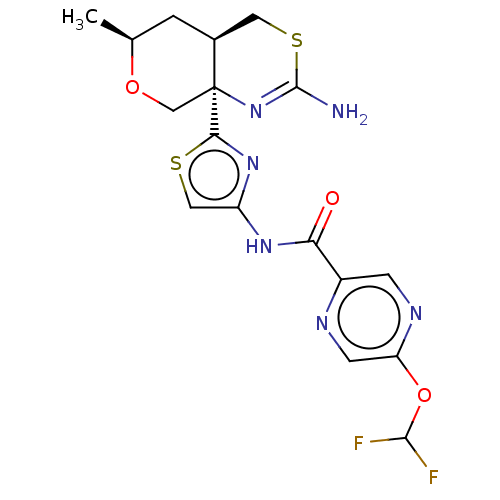 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259963 (CHEMBL4061944) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223396 (US9315520, 7 | US9315520, Example 7 | US9605007, E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50337556 (6-(4-hydroxy-4-phenylpiperidin-1-yl)-1-methyl-4-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta expressed in baculovirus infected SF9 cells after 60 mins | Bioorg Med Chem Lett 21: 1429-33 (2011) Article DOI: 10.1016/j.bmcl.2011.01.017 BindingDB Entry DOI: 10.7270/Q2KH0NNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223400 (US9315520, 45 | US9315520, Example 45 | US9605007,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452877 (CHEMBL4205912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 357 total ) | Next | Last >> |