

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of liver TDO | Eur J Med Chem 54: 95-102 (2012) Article DOI: 10.1016/j.ejmech.2012.04.033 BindingDB Entry DOI: 10.7270/Q2XS5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50350281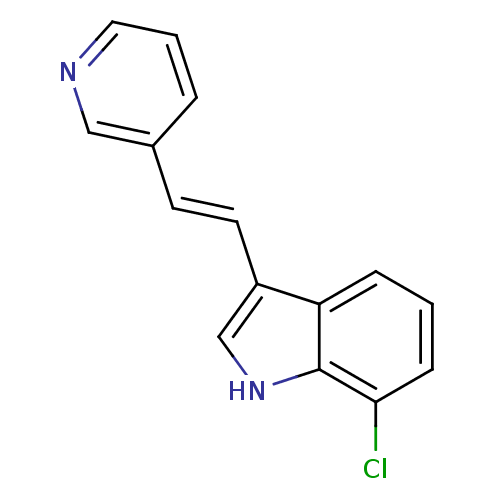 (CHEMBL1812527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Competitive inhibition of human purified TDO by Henri-Michaelis-Menten equation analysis | Eur J Med Chem 54: 95-102 (2012) Article DOI: 10.1016/j.ejmech.2012.04.033 BindingDB Entry DOI: 10.7270/Q2XS5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470233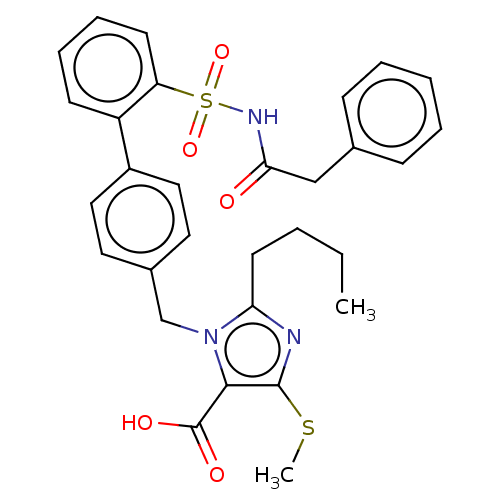 (CHEMBL80177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470229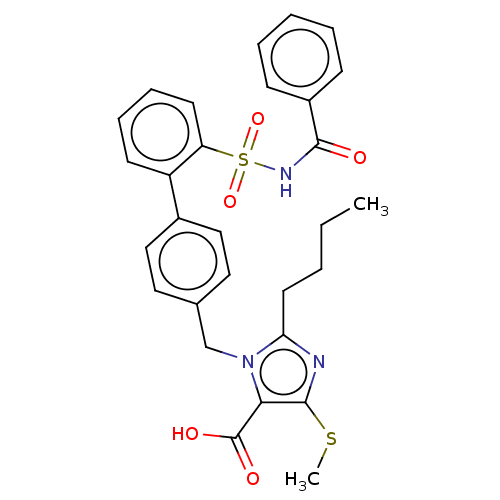 (CHEMBL76870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470236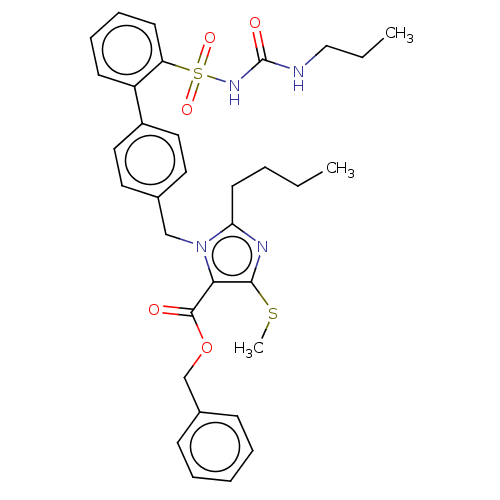 (CHEMBL309313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-5/beta-1 (Homo sapiens (Human)) | BDBM50313793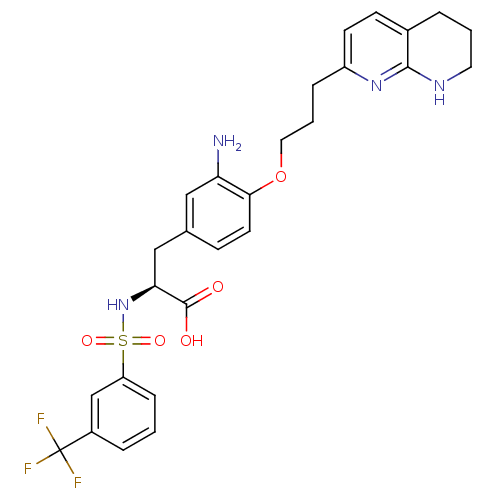 ((S)-3-(3-amino-4-(3-(5,6,7,8-tetrahydro-1,8-naphth...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human Integrin alpha5beta3 receptors expressed in HOP cells assessed as cell attachment after 30 mins by colorimetric assay | Bioorg Med Chem Lett 20: 1861-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.150 BindingDB Entry DOI: 10.7270/Q2Z60P6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470239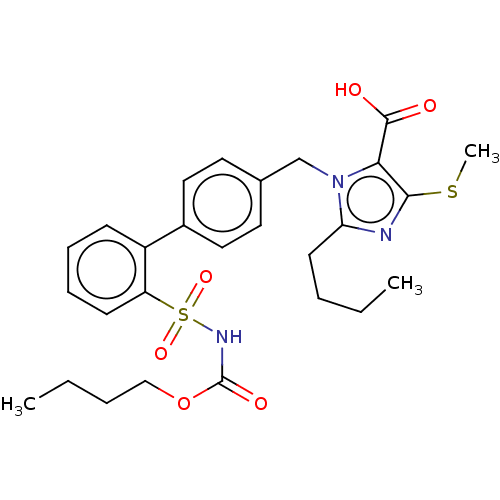 (CHEMBL311312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470206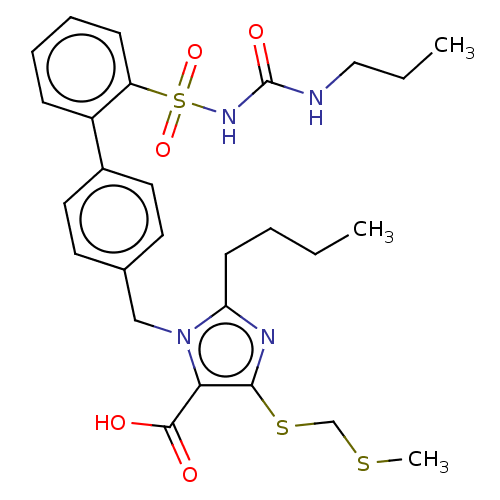 (CHEMBL445365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470250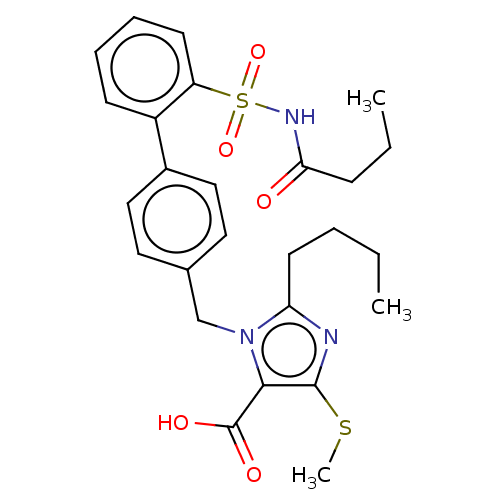 (CHEMBL77308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213557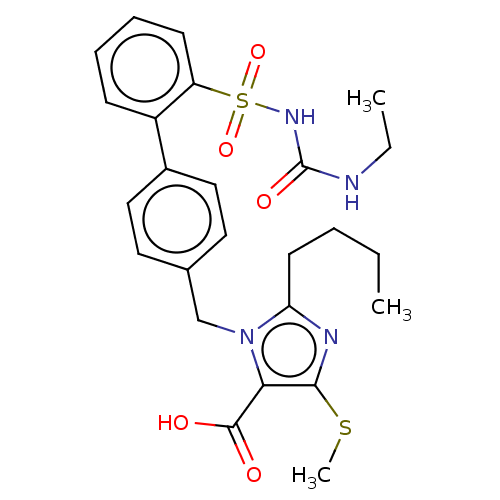 (CHEMBL309602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031491 (2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470225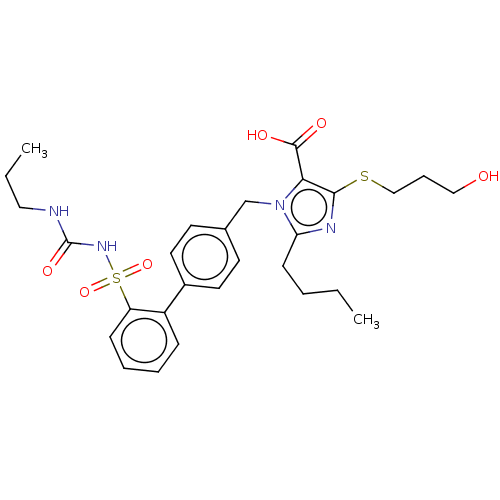 (CHEMBL80700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470235 (CHEMBL308135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470226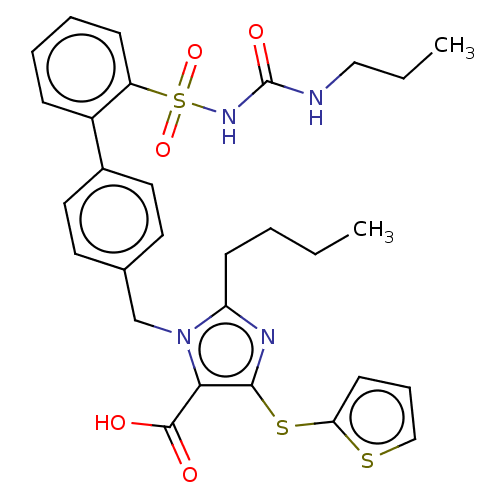 (CHEMBL311827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213569 (CHEMBL78866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470241 (CHEMBL305638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM50011716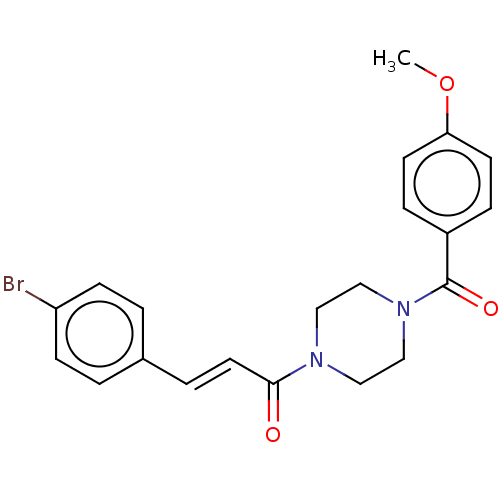 (CHEMBL3262896) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at EBI2 receptor in human U937 cells assessed as inhibition of 7-alpha,25-OHC-induced cell migration after 3 hrs by flow cytometr... | J Med Chem 57: 3358-68 (2014) Article DOI: 10.1021/jm4019355 BindingDB Entry DOI: 10.7270/Q2BG2QJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470223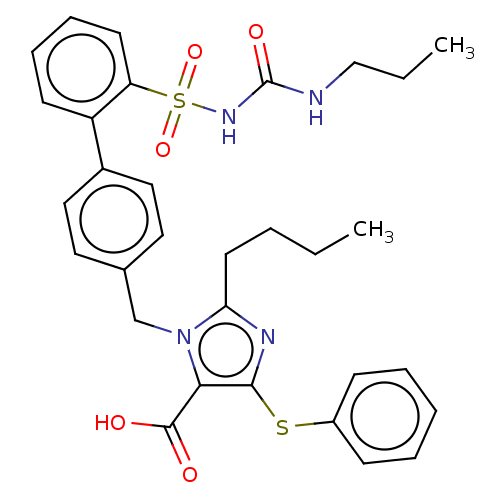 (CHEMBL312386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031537 (2-Butyl-3-(N'-propylureidylsulfonyl-biphenyl-4-ylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-5/beta-1 (Homo sapiens (Human)) | BDBM50313795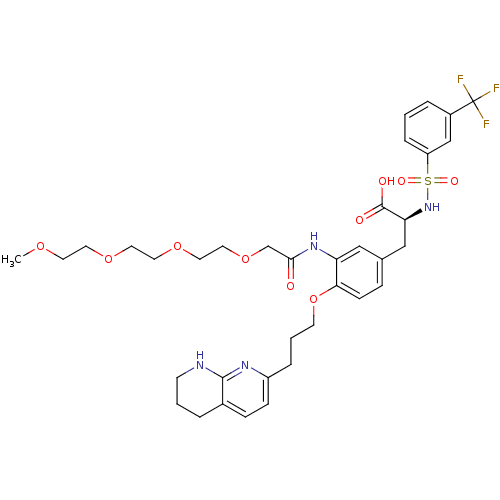 ((S)-3-(4-(3-(5,6,7,8-tetrahydro-1,8-naphthyridin-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human Integrin alpha5beta3 receptors expressed in HOP cells assessed as cell attachment after 30 mins by colorimetric assay | Bioorg Med Chem Lett 20: 1861-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.150 BindingDB Entry DOI: 10.7270/Q2Z60P6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470249 (CHEMBL309845) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470227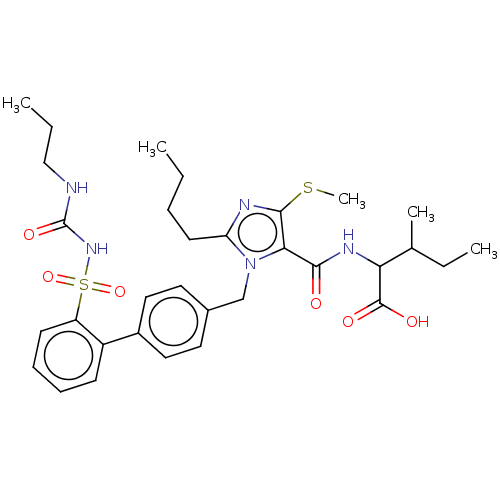 (CHEMBL76538) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470228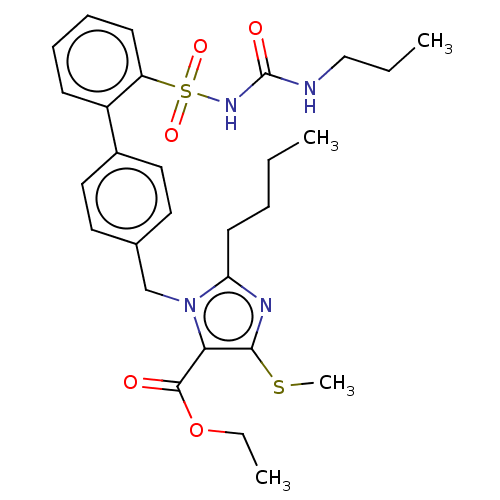 (CHEMBL80971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470234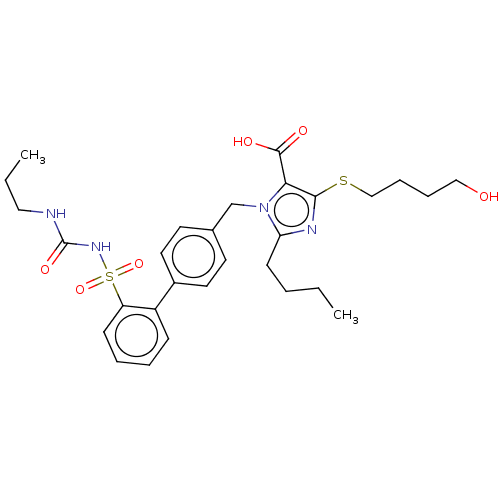 (CHEMBL76948) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031530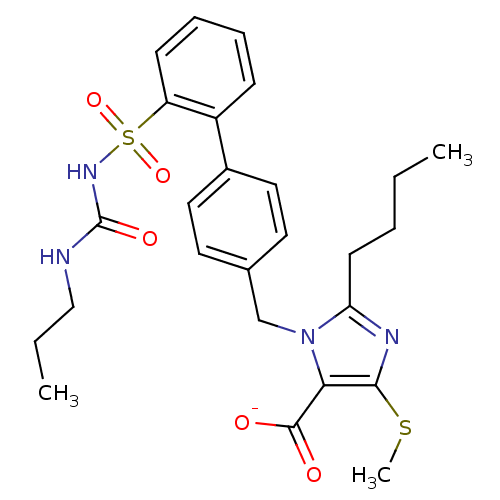 (2-Butyl-4-(methylthio)-1-[[2'-[[[(propylamino)carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470243 (CHEMBL306157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470219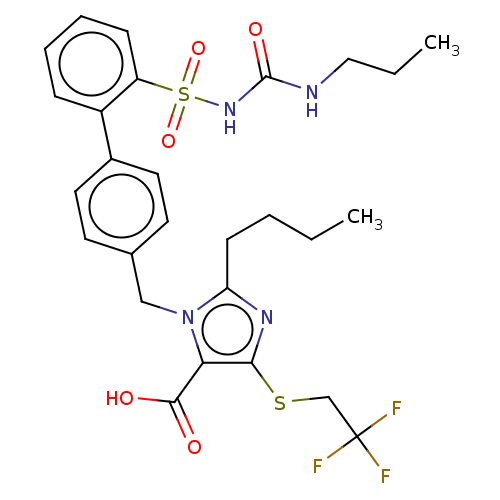 (CHEMBL305604) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470212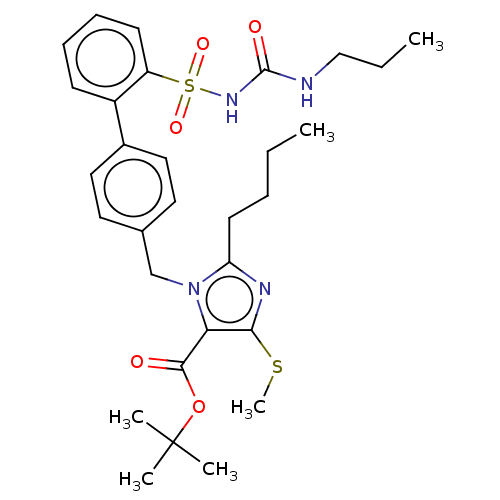 (CHEMBL80622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-5/beta-1 (Homo sapiens (Human)) | BDBM50313794 ((S)-3-(3-(20-amino-3,6,9,12,15,18-hexaoxaicosanami...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human Integrin alpha5beta3 receptors expressed in HOP cells assessed as cell attachment after 30 mins by colorimetric assay | Bioorg Med Chem Lett 20: 1861-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.150 BindingDB Entry DOI: 10.7270/Q2Z60P6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470240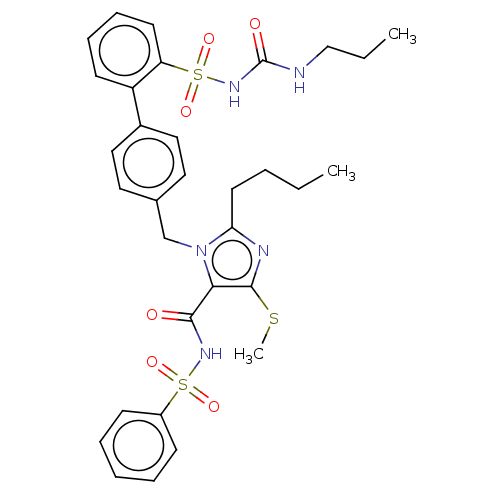 (CHEMBL432158) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470217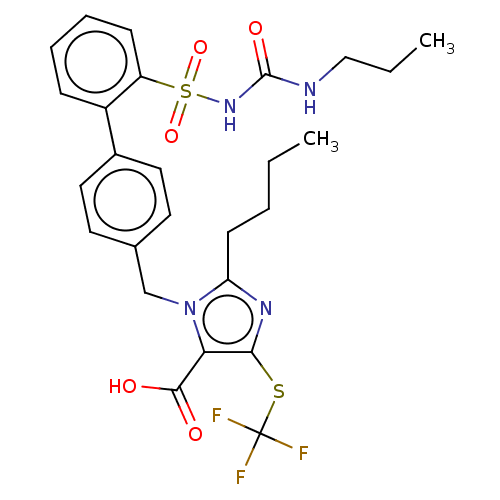 (CHEMBL77138) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470214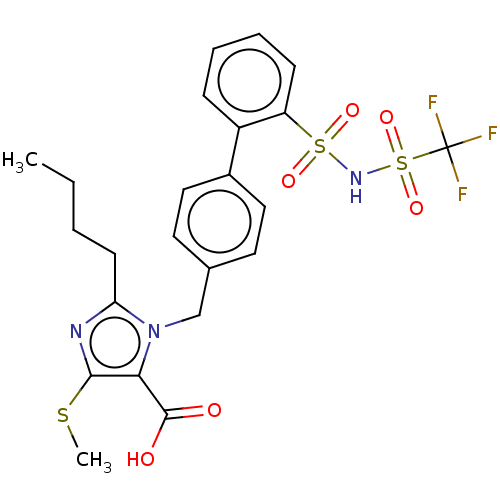 (CHEMBL77360) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470230 (CHEMBL76799) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50093978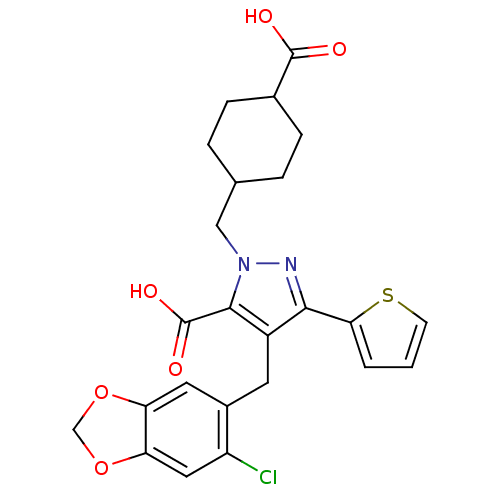 (2-(4-Carboxy-cyclohexylmethyl)-4-(6-chloro-benzo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin A receptor in rat heart ventricles | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470248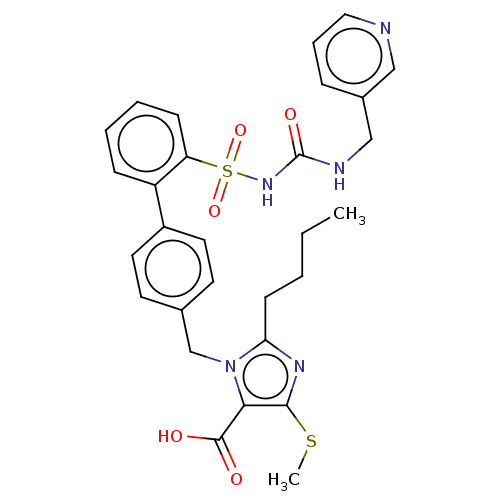 (CHEMBL311847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470224 (CHEMBL311189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470208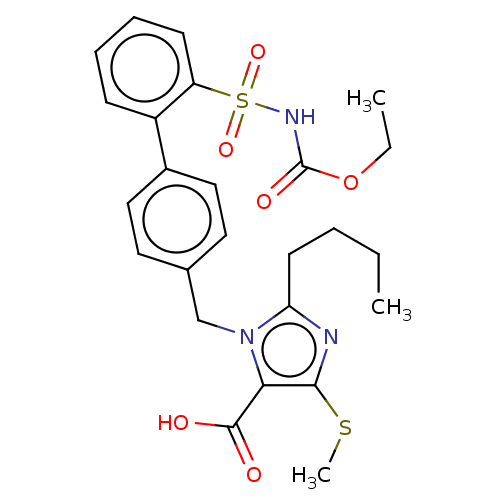 (CHEMBL80900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470246 (CHEMBL306085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470237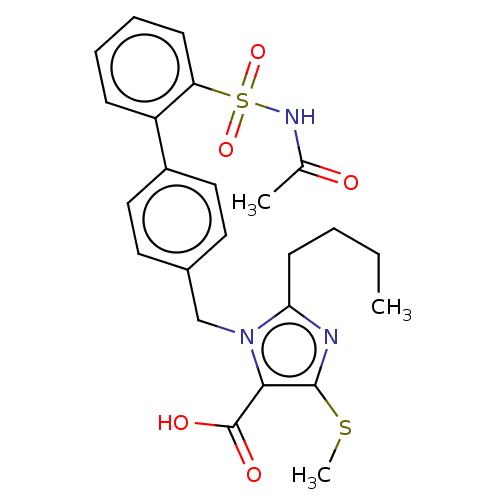 (CHEMBL306778) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470231 (CHEMBL76613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50093997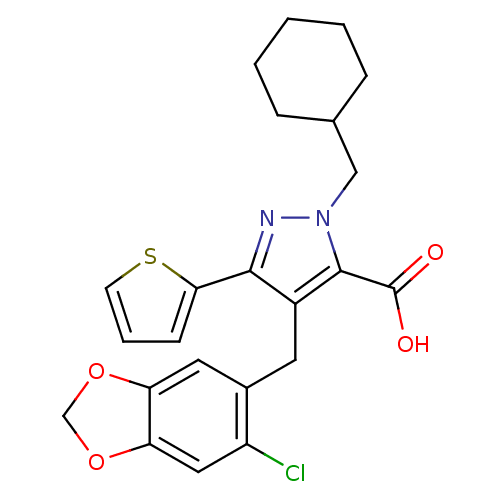 (4-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-2-cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin A receptor in rat heart ventricles | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50093996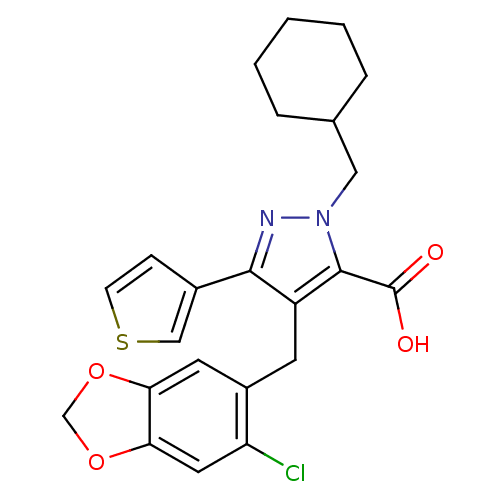 (4-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-2-cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin A receptor in rat heart ventricles | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470222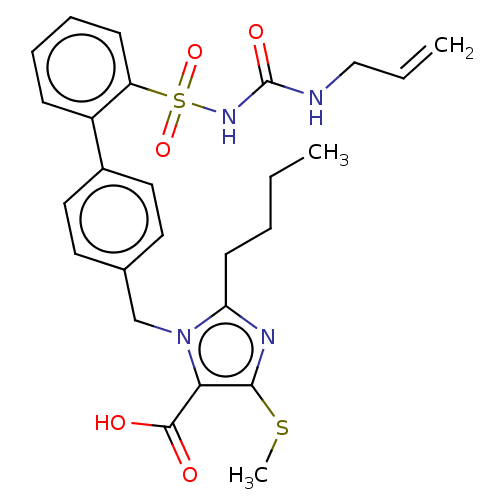 (CHEMBL443069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470238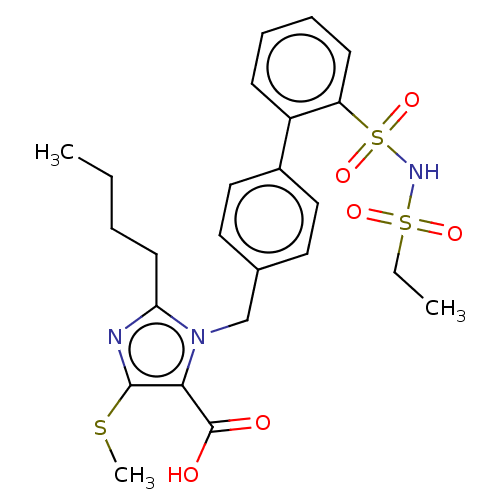 (CHEMBL80908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50094000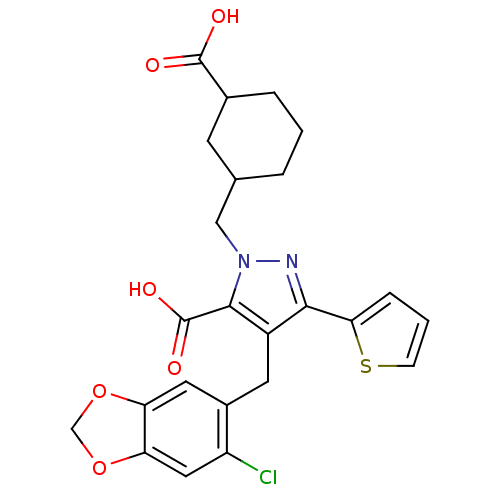 (2-(3-Carboxy-cyclohexylmethyl)-4-(6-chloro-benzo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin A receptor in rat heart ventricles | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50093987 (4-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-2-cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin A receptor in rat heart ventricles | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470216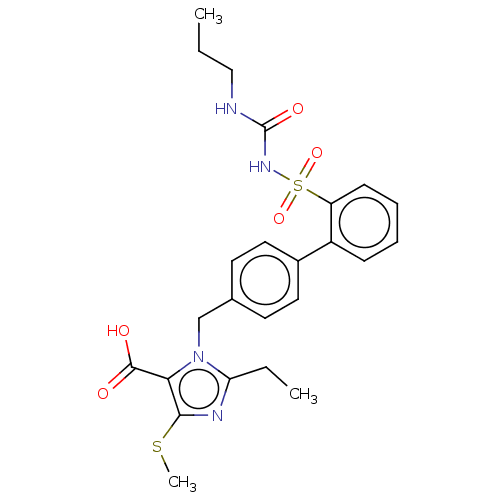 (CHEMBL310267) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470245 (CHEMBL78169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50093997 (4-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-2-cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by ChEMBL | Assay Description In vitro binding affinity to endothelin B receptor in rat cerebellum | Bioorg Med Chem Lett 10: 2575-8 (2001) BindingDB Entry DOI: 10.7270/Q22R3QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470207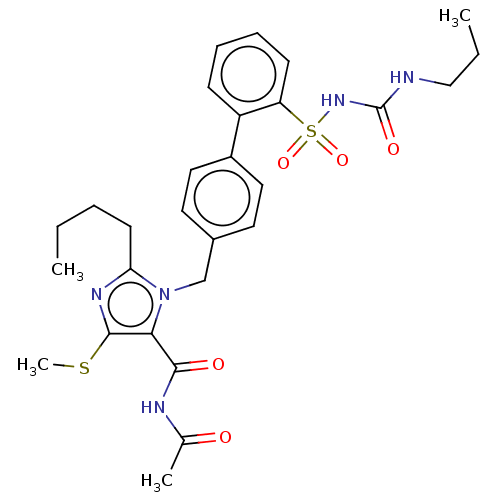 (CHEMBL413491) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |