

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal CYP2D6 using {3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin} as substrate assessed as ... | J Med Chem 56: 7851-61 (2013) Article DOI: 10.1021/jm400766k BindingDB Entry DOI: 10.7270/Q290257C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272209 (1-((9H-Fluoren-2-yl)ethyl)-1H-imidazole | CHEMBL50...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of CYP17 (unknown origin) | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295628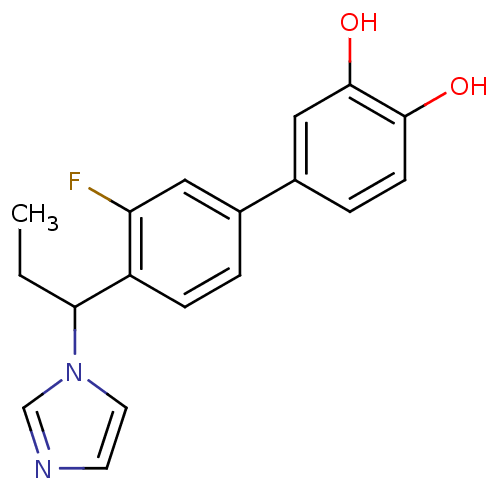 (CHEMBL571003 | rac-3'-Fluoro-4'-(1-imidazol-1-yl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25457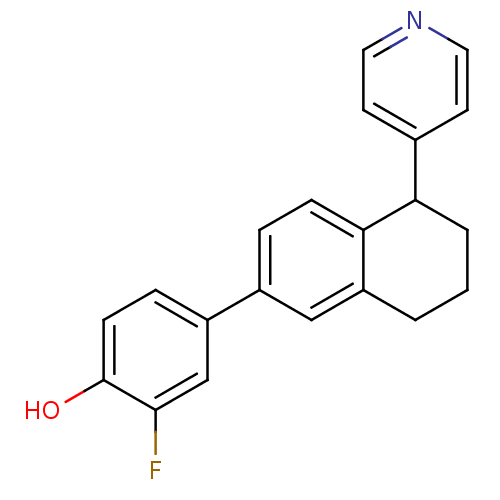 (2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295627 (CHEMBL556280 | rac-3'-Fluoro-4'-(1-imidazol-1-yl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50295632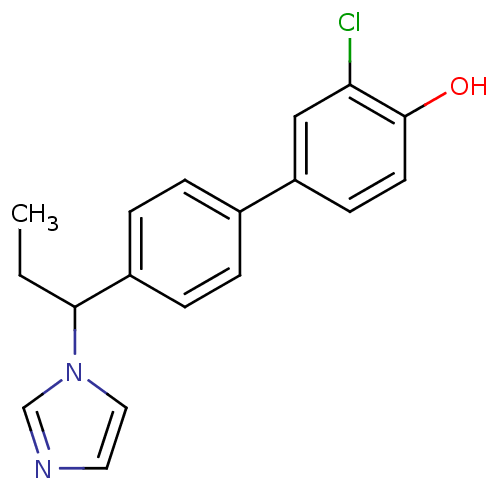 (CHEMBL541941 | rac-3-Chloro-4'-(1-imidazol-1-yl-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 expressed in baculovirus-infected insect microsome at 1 uM | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272156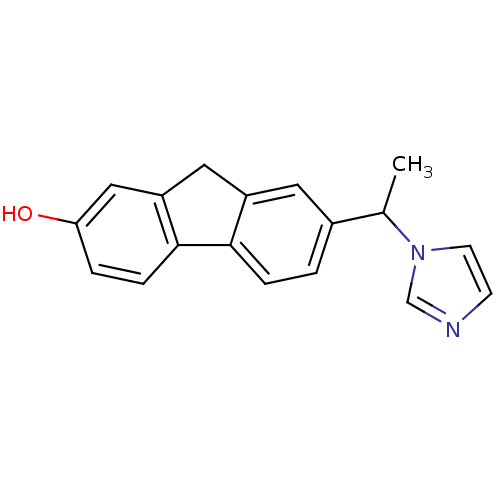 (7-(1-(1H-imidazol-1-yl)ethyl)-9H-fluoren-2-ol | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272209 (1-((9H-Fluoren-2-yl)ethyl)-1H-imidazole | CHEMBL50...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272212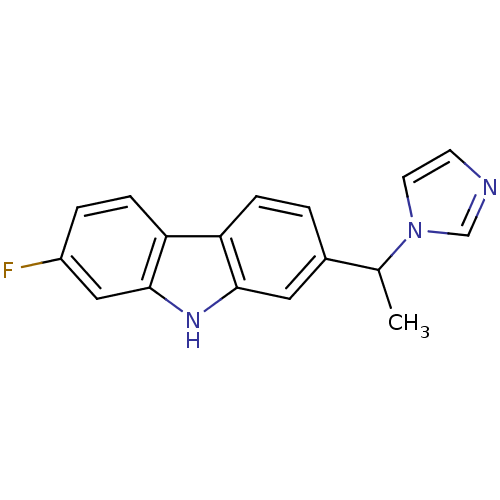 (2-(1-(1H-Imidazol-1-yl)ethyl)-7-fluoro-9H-carbazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25448 (4-[6-(4-fluorophenyl)-3,4-dihydronaphthalen-1-yl]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25456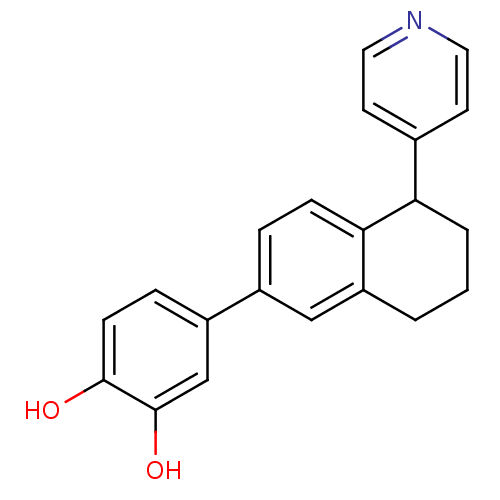 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295625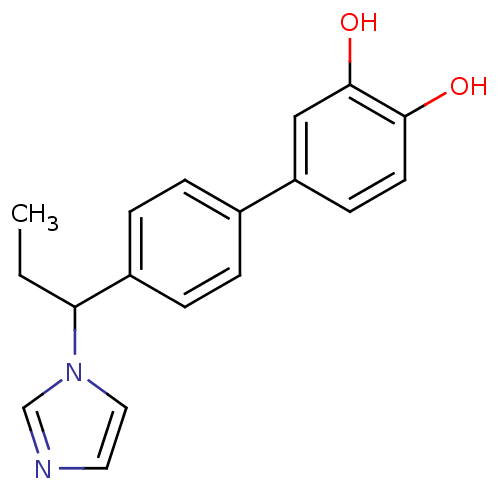 (CHEMBL551253 | rac-4'-(1-Imidazol-1-yl-propyl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25446 (4-[6-(4-fluorophenyl)-1H-inden-3-yl]pyridine | Abi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25454 (4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295624 (CHEMBL556833 | rac-4'-(1-Imidazol-1-yl-propyl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272210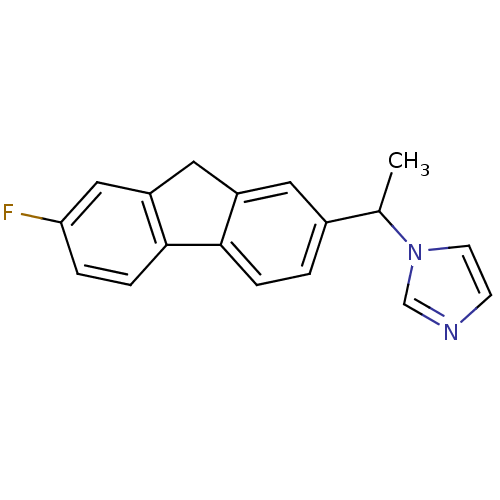 (1-[1-(7-Fluoro-9H-fluoren-2-yl)-ethyl]-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295629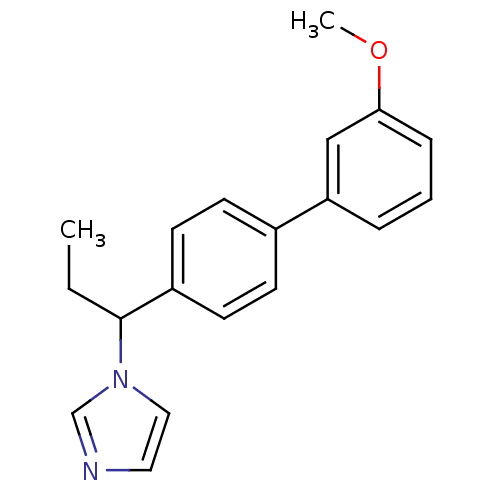 (CHEMBL540997 | rac-1-[1-(3'-Methoxy-biphenyl-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295626 (CHEMBL565098 | rac-4'-(1-Imidazol-1-yl-propyl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295632 (CHEMBL541941 | rac-3-Chloro-4'-(1-imidazol-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat testicular CYP17 | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272067 (4'-(1H -Imidazol-1-yl-propyl)-biphenyl-4-ol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25453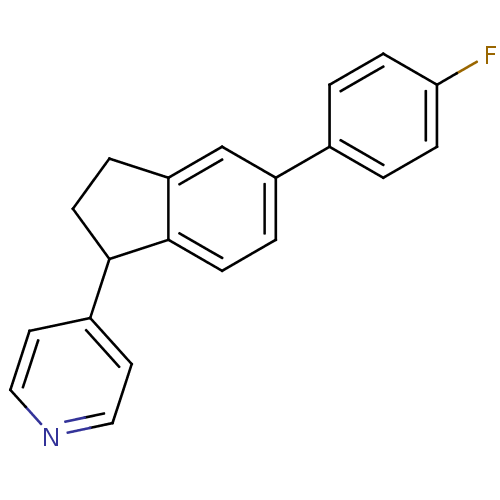 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50371480 (CHEMBL403808) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295631 (CHEMBL557233 | rac-4'-(1-Imidazol-1-yl-propyl)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50371479 (CHEMBL403852) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272211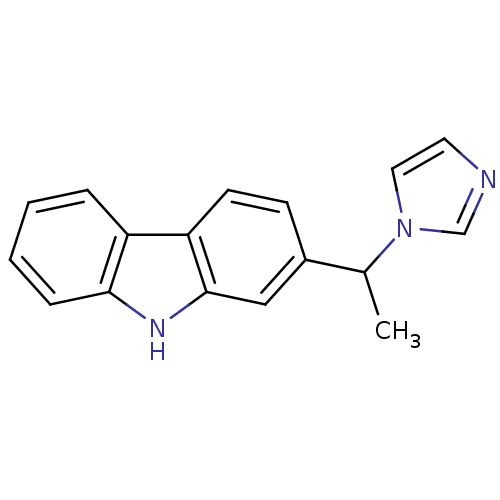 (2-(1-Imidazol-1-yl-ethyl)-9H-carbazole | CHEMBL499...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25441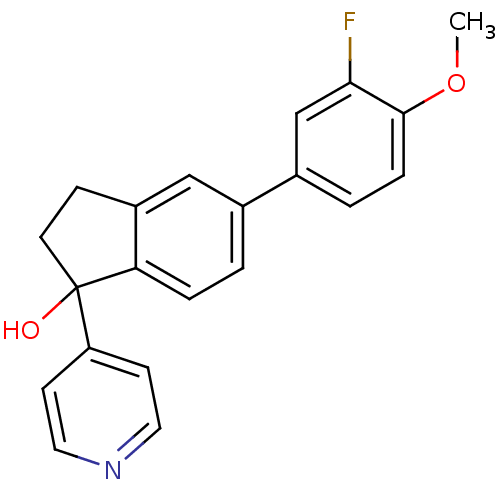 (5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272542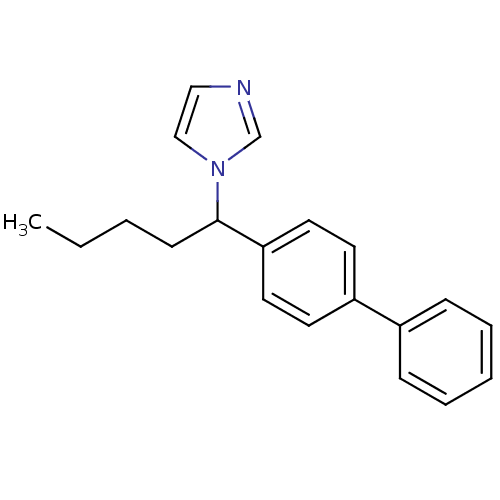 (1-(1-Biphenyl-4-yl-pentyl)-1H-imidazole | CHEMBL49...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25450 (4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 307 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272541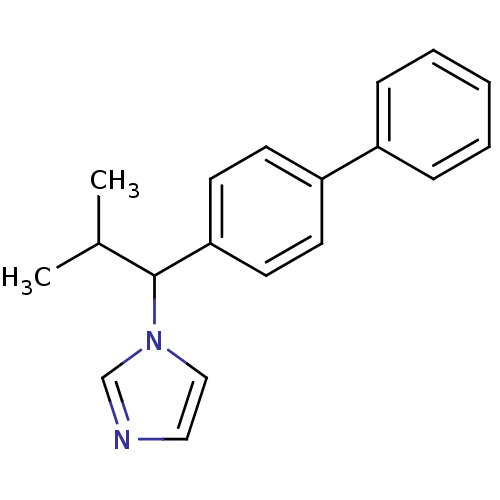 (1-(1-Biphenyl-4-yl-2-methyl-propyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25449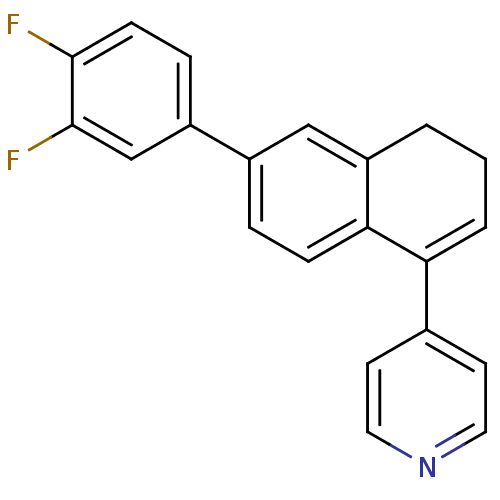 (4-[6-(3,4-difluorophenyl)-3,4-dihydronaphthalen-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25439 (5-(4-fluorophenyl)-1-(pyridin-4-yl)-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 333 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272068 (1-[1-(4'-Fluoro-biphenyl-4-yl)-propyl]-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25450 (4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50371478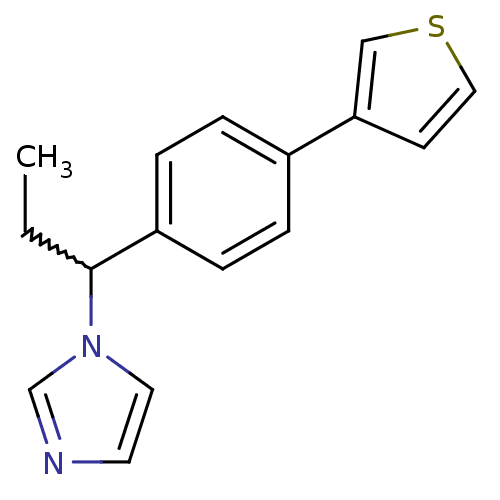 (CHEMBL403475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272067 (4'-(1H -Imidazol-1-yl-propyl)-biphenyl-4-ol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50295630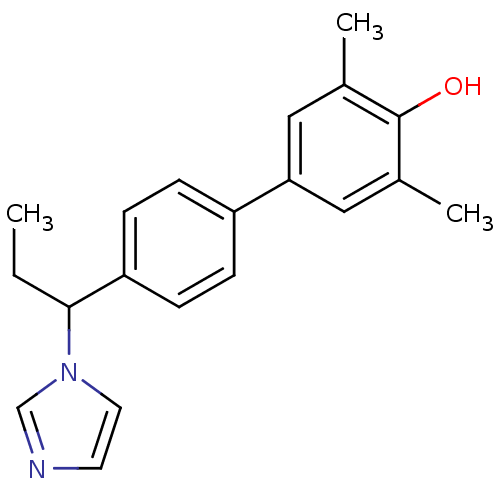 (CHEMBL557232 | rac-4'-(1-Imidazol-1-yl-propyl)-3,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase | Eur J Med Chem 44: 2765-75 (2009) Article DOI: 10.1016/j.ejmech.2009.01.002 BindingDB Entry DOI: 10.7270/Q24B31BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272157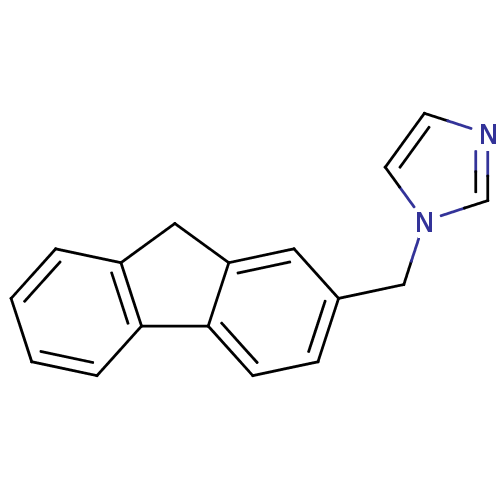 (1-((9H-Fluoren-2-yl)methyl)-1H-imidazole | CHEMBL5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25443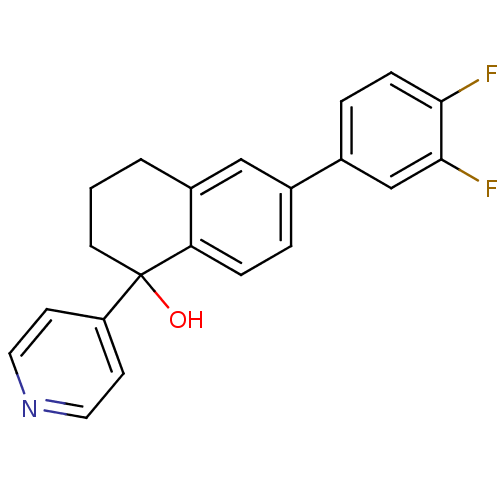 (6-(3,4-difluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 423 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25441 (5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 436 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272507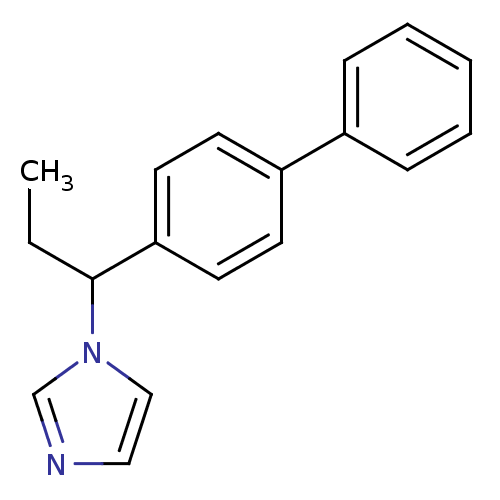 (1-(1-Biphenyl-4-yl-propyl)-1H-imidazole | CHEMBL49...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50272544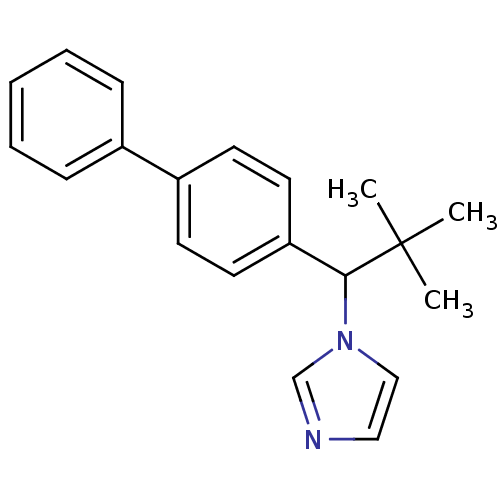 (1-(1-Biphenyl-4-yl-2,2-dimethyl-propyl)-1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductase | Bioorg Med Chem 16: 7715-27 (2008) Article DOI: 10.1016/j.bmc.2008.07.011 BindingDB Entry DOI: 10.7270/Q2222TK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |