 Found 113 hits with Last Name = 'le' and Initial = 'tv'
Found 113 hits with Last Name = 'le' and Initial = 'tv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289438
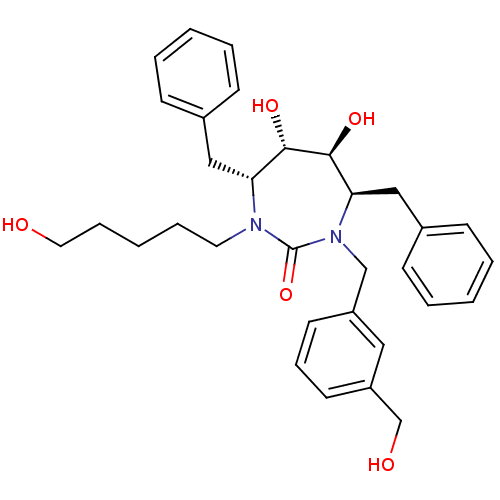
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(3-hydr...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(CO)c2)C1=O Show InChI InChI=1S/C32H40N2O5/c35-18-9-3-8-17-33-28(20-24-11-4-1-5-12-24)30(37)31(38)29(21-25-13-6-2-7-14-25)34(32(33)39)22-26-15-10-16-27(19-26)23-36/h1-2,4-7,10-16,19,28-31,35-38H,3,8-9,17-18,20-23H2/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289443
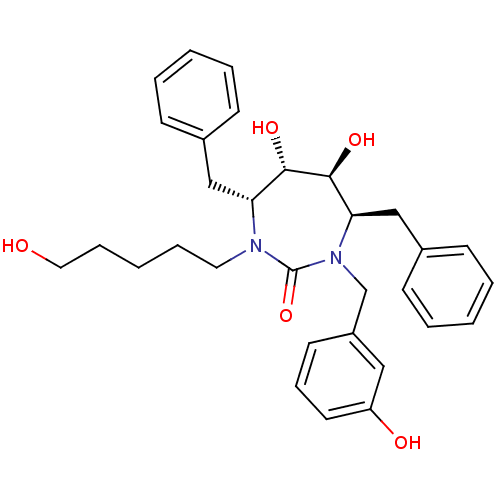
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(3-hydr...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(O)c2)C1=O Show InChI InChI=1S/C31H38N2O5/c34-18-9-3-8-17-32-27(20-23-11-4-1-5-12-23)29(36)30(37)28(21-24-13-6-2-7-14-24)33(31(32)38)22-25-15-10-16-26(35)19-25/h1-2,4-7,10-16,19,27-30,34-37H,3,8-9,17-18,20-22H2/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289441
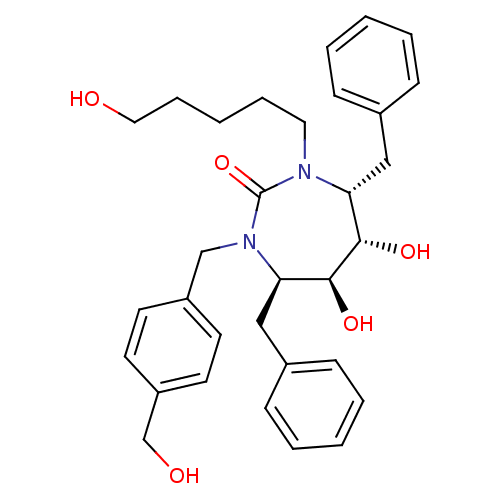
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(4-hydr...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc(CO)cc2)C1=O Show InChI InChI=1S/C32H40N2O5/c35-19-9-3-8-18-33-28(20-24-10-4-1-5-11-24)30(37)31(38)29(21-25-12-6-2-7-13-25)34(32(33)39)22-26-14-16-27(23-36)17-15-26/h1-2,4-7,10-17,28-31,35-38H,3,8-9,18-23H2/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289445
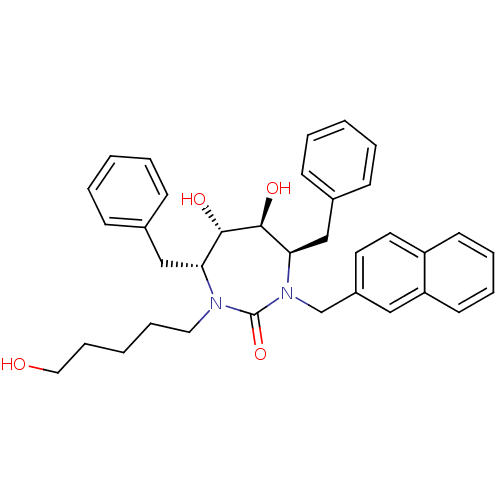
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(5-hydr...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3ccccc3c2)C1=O Show InChI InChI=1S/C35H40N2O4/c38-21-11-3-10-20-36-31(23-26-12-4-1-5-13-26)33(39)34(40)32(24-27-14-6-2-7-15-27)37(35(36)41)25-28-18-19-29-16-8-9-17-30(29)22-28/h1-2,4-9,12-19,22,31-34,38-40H,3,10-11,20-21,23-25H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM21
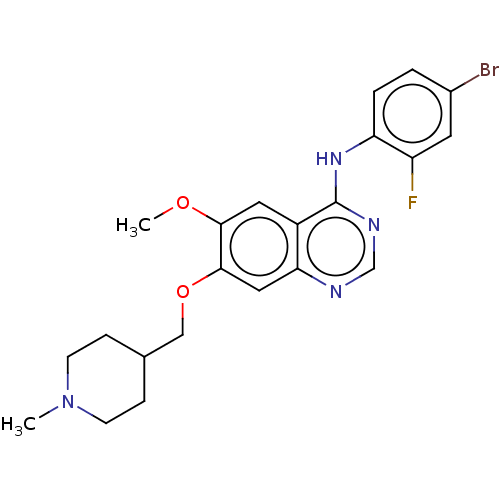
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM150
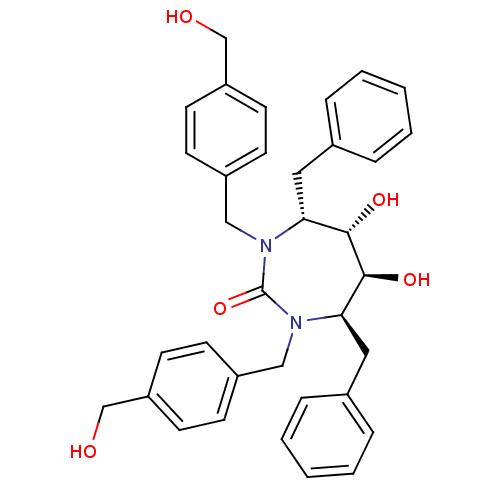
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES OCc1ccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc(CO)cc3)C2=O)cc1 Show InChI InChI=1S/C35H38N2O5/c38-23-29-15-11-27(12-16-29)21-36-31(19-25-7-3-1-4-8-25)33(40)34(41)32(20-26-9-5-2-6-10-26)37(35(36)42)22-28-13-17-30(24-39)18-14-28/h1-18,31-34,38-41H,19-24H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM33

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(6...)Show SMILES OCCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCCO)C1=O Show InChI InChI=1S/C31H46N2O5/c34-21-13-3-1-11-19-32-27(23-25-15-7-5-8-16-25)29(36)30(37)28(24-26-17-9-6-10-18-26)33(31(32)38)20-12-2-4-14-22-35/h5-10,15-18,27-30,34-37H,1-4,11-14,19-24H2/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289440
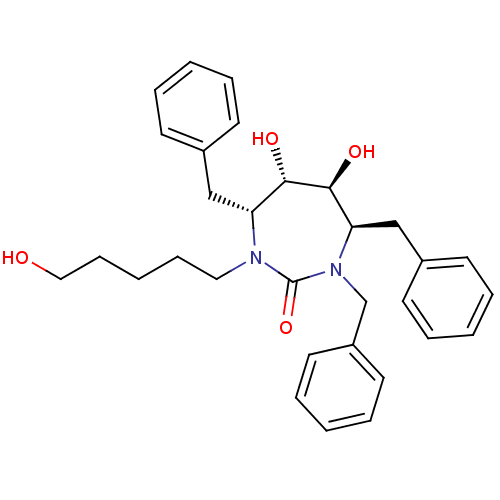
((4R,5S,6S,7R)-1,4,7-Tribenzyl-5,6-dihydroxy-3-(5-h...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccccc2)C1=O Show InChI InChI=1S/C31H38N2O4/c34-20-12-4-11-19-32-27(21-24-13-5-1-6-14-24)29(35)30(36)28(22-25-15-7-2-8-16-25)33(31(32)37)23-26-17-9-3-10-18-26/h1-3,5-10,13-18,27-30,34-36H,4,11-12,19-23H2/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289437
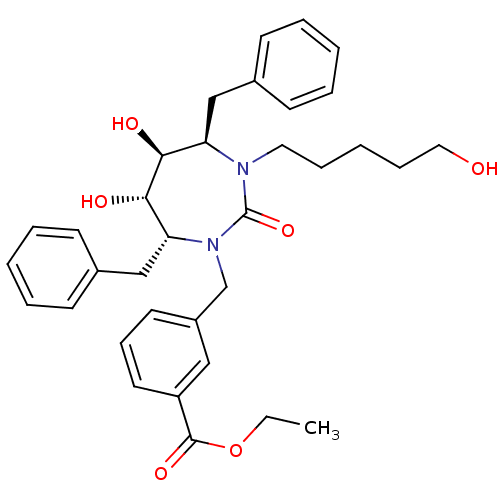
(3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(5-h...)Show SMILES CCOC(=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(CCCCCO)C2=O)c1 Show InChI InChI=1S/C34H42N2O6/c1-2-42-33(40)28-18-12-17-27(21-28)24-36-30(23-26-15-8-4-9-16-26)32(39)31(38)29(22-25-13-6-3-7-14-25)35(34(36)41)19-10-5-11-20-37/h3-4,6-9,12-18,21,29-32,37-39H,2,5,10-11,19-20,22-24H2,1H3/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM25

((4R,5S,6S,7R)-4,7-dibenzyl-1-(cyclopropylmethyl)-5...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CC2CC2)C1=O Show InChI InChI=1S/C28H38N2O4/c31-17-9-3-8-16-29-24(18-21-10-4-1-5-11-21)26(32)27(33)25(19-22-12-6-2-7-13-22)30(28(29)34)20-23-14-15-23/h1-2,4-7,10-13,23-27,31-33H,3,8-9,14-20H2/t24-,25-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM28
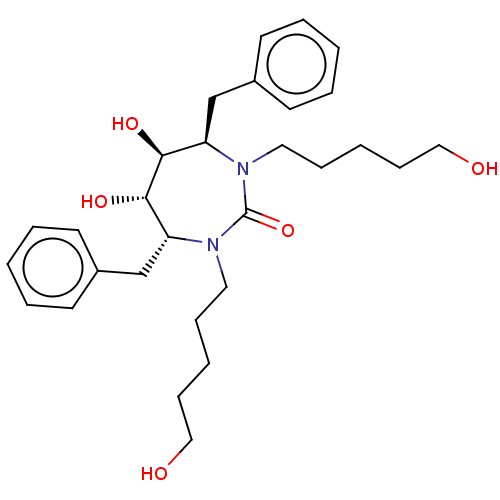
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(5...)Show SMILES OCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCO)C1=O Show InChI InChI=1S/C29H42N2O5/c32-19-11-3-9-17-30-25(21-23-13-5-1-6-14-23)27(34)28(35)26(22-24-15-7-2-8-16-24)31(29(30)36)18-10-4-12-20-33/h1-2,5-8,13-16,25-28,32-35H,3-4,9-12,17-22H2/t25-,26-,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289439
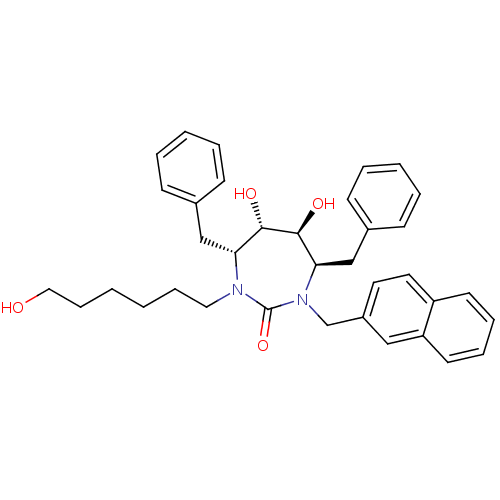
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(6-hydr...)Show SMILES OCCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3ccccc3c2)C1=O Show InChI InChI=1S/C36H42N2O4/c39-22-12-2-1-11-21-37-32(24-27-13-5-3-6-14-27)34(40)35(41)33(25-28-15-7-4-8-16-28)38(36(37)42)26-29-19-20-30-17-9-10-18-31(30)23-29/h3-10,13-20,23,32-35,39-41H,1-2,11-12,21-22,24-26H2/t32-,33-,34+,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289446

(CHEMBL280989 | N-(5-{(4R,5S,6S,7R)-4,7-Dibenzyl-5,...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)Cc2cccnc2)C(=O)N(CCCCCNC(=O)Cc2cccnc2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H54N6O5/c50-39(29-35-19-13-21-44-31-35)46-23-9-3-11-25-48-37(27-33-15-5-1-6-16-33)41(52)42(53)38(28-34-17-7-2-8-18-34)49(43(48)54)26-12-4-10-24-47-40(51)30-36-20-14-22-45-32-36/h1-2,5-8,13-22,31-32,37-38,41-42,52-53H,3-4,9-12,23-30H2,(H,46,50)(H,47,51)/t37-,38-,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM67
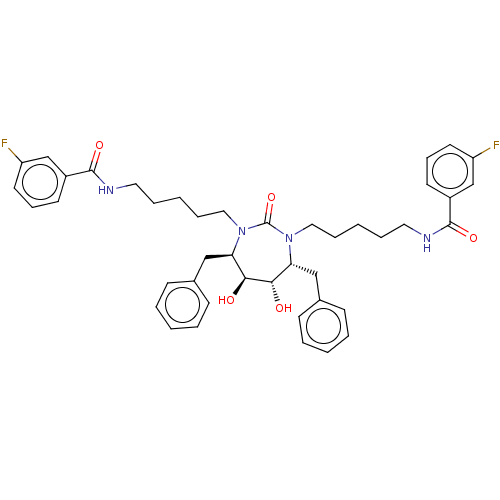
(DMPC Cyclic Urea 47 | N-{5-[(4R,5S,6S,7R)-4,7-dibe...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)c2cccc(F)c2)C(=O)N(CCCCCNC(=O)c2cccc(F)c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H50F2N4O5/c44-35-21-13-19-33(29-35)41(52)46-23-9-3-11-25-48-37(27-31-15-5-1-6-16-31)39(50)40(51)38(28-32-17-7-2-8-18-32)49(43(48)54)26-12-4-10-24-47-42(53)34-20-14-22-36(45)30-34/h1-2,5-8,13-22,29-30,37-40,50-51H,3-4,9-12,23-28H2,(H,46,52)(H,47,53)/t37-,38-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM69
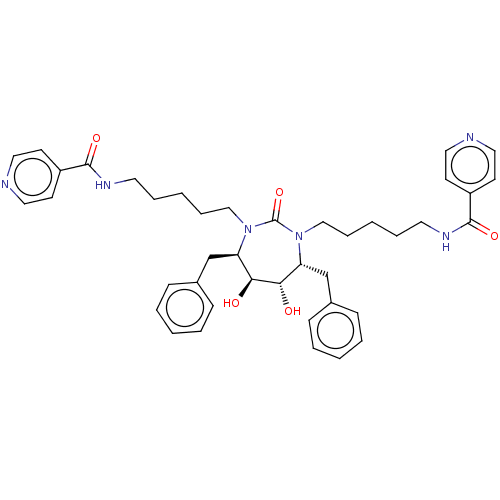
(CHEMBL282395 | DMPC Cyclic Urea 49 | N-{5-[(4R,5S,...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)c2ccncc2)C(=O)N(CCCCCNC(=O)c2ccncc2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H50N6O5/c48-37-35(29-31-13-5-1-6-14-31)46(27-11-3-9-21-44-39(50)33-17-23-42-24-18-33)41(52)47(36(38(37)49)30-32-15-7-2-8-16-32)28-12-4-10-22-45-40(51)34-19-25-43-26-20-34/h1-2,5-8,13-20,23-26,35-38,48-49H,3-4,9-12,21-22,27-30H2,(H,44,50)(H,45,51)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM70
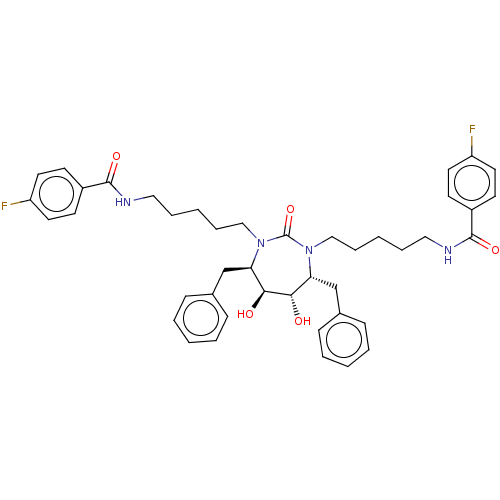
(DMPC Cyclic Urea 50 | N-{5-[(4R,5S,6S,7R)-4,7-dibe...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)c2ccc(F)cc2)C(=O)N(CCCCCNC(=O)c2ccc(F)cc2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H50F2N4O5/c44-35-21-17-33(18-22-35)41(52)46-25-9-3-11-27-48-37(29-31-13-5-1-6-14-31)39(50)40(51)38(30-32-15-7-2-8-16-32)49(43(48)54)28-12-4-10-26-47-42(53)34-19-23-36(45)24-20-34/h1-2,5-8,13-24,37-40,50-51H,3-4,9-12,25-30H2,(H,46,52)(H,47,53)/t37-,38-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM76
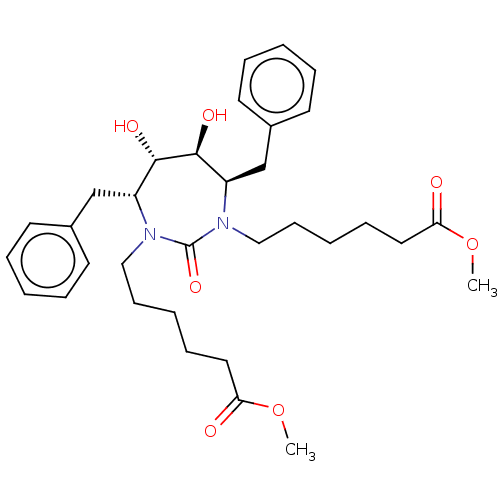
(DMPC Cyclic Urea 56 | methyl 6-[(4R,5S,6S,7R)-4,7-...)Show SMILES COC(=O)CCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCC(=O)OC)C1=O Show InChI InChI=1S/C33H46N2O7/c1-41-29(36)19-11-5-13-21-34-27(23-25-15-7-3-8-16-25)31(38)32(39)28(24-26-17-9-4-10-18-26)35(33(34)40)22-14-6-12-20-30(37)42-2/h3-4,7-10,15-18,27-28,31-32,38-39H,5-6,11-14,19-24H2,1-2H3/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM81

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(7...)Show SMILES OCCCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCCCO)C1=O Show InChI InChI=1S/C33H50N2O5/c36-23-15-5-1-3-13-21-34-29(25-27-17-9-7-10-18-27)31(38)32(39)30(26-28-19-11-8-12-20-28)35(33(34)40)22-14-4-2-6-16-24-37/h7-12,17-20,29-32,36-39H,1-6,13-16,21-26H2/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM87
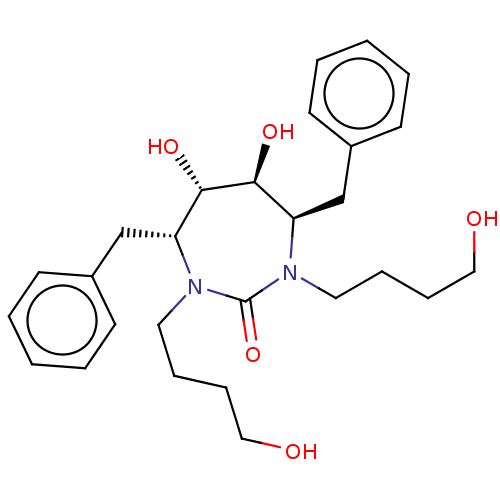
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(4...)Show SMILES OCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCO)C1=O Show InChI InChI=1S/C27H38N2O5/c30-17-9-7-15-28-23(19-21-11-3-1-4-12-21)25(32)26(33)24(20-22-13-5-2-6-14-22)29(27(28)34)16-8-10-18-31/h1-6,11-14,23-26,30-33H,7-10,15-20H2/t23-,24-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM92
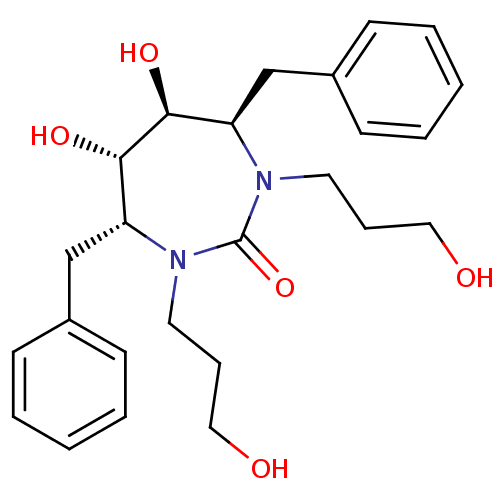
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(3...)Show SMILES OCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCO)C1=O Show InChI InChI=1S/C25H34N2O5/c28-15-7-13-26-21(17-19-9-3-1-4-10-19)23(30)24(31)22(18-20-11-5-2-6-12-20)27(25(26)32)14-8-16-29/h1-6,9-12,21-24,28-31H,7-8,13-18H2/t21-,22-,23+,24+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM93
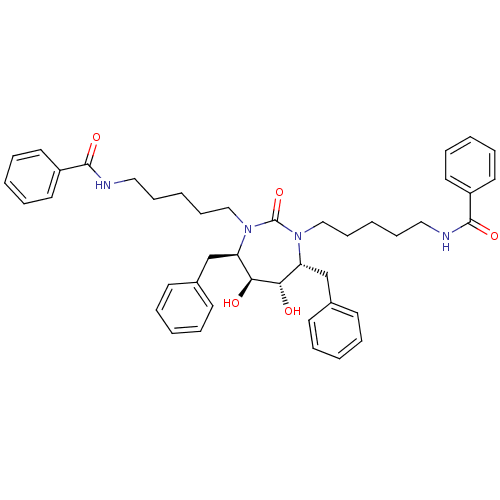
(DMPC Cyclic Urea 73 | N-{5-[(4R,5S,6S,7R)-4,7-dibe...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)c2ccccc2)C(=O)N(CCCCCNC(=O)c2ccccc2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H52N4O5/c48-39-37(31-33-19-7-1-8-20-33)46(29-17-5-15-27-44-41(50)35-23-11-3-12-24-35)43(52)47(38(40(39)49)32-34-21-9-2-10-22-34)30-18-6-16-28-45-42(51)36-25-13-4-14-26-36/h1-4,7-14,19-26,37-40,48-49H,5-6,15-18,27-32H2,(H,44,50)(H,45,51)/t37-,38-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM98
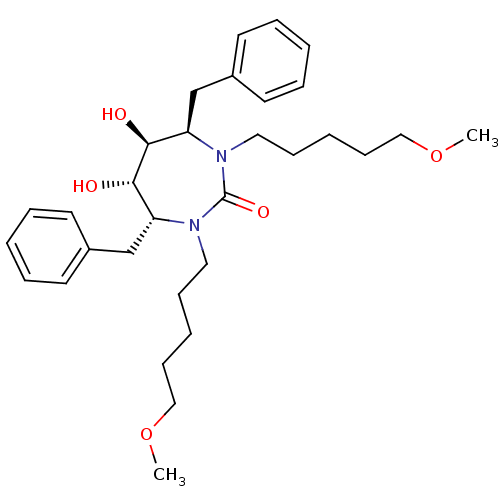
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(5...)Show SMILES COCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCOC)C1=O Show InChI InChI=1S/C31H46N2O5/c1-37-21-13-5-11-19-32-27(23-25-15-7-3-8-16-25)29(34)30(35)28(24-26-17-9-4-10-18-26)33(31(32)36)20-12-6-14-22-38-2/h3-4,7-10,15-18,27-30,34-35H,5-6,11-14,19-24H2,1-2H3/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM114

(1-{5-[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{...)Show SMILES CNC(=O)NCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(=O)NC)C1=O Show InChI InChI=1S/C33H50N6O5/c1-34-31(42)36-19-11-5-13-21-38-27(23-25-15-7-3-8-16-25)29(40)30(41)28(24-26-17-9-4-10-18-26)39(33(38)44)22-14-6-12-20-37-32(43)35-2/h3-4,7-10,15-18,27-30,40-41H,5-6,11-14,19-24H2,1-2H3,(H2,34,36,42)(H2,35,37,43)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM108

(DMPC Cyclic Urea 88 | N-{5-[(4R,5S,6S,7R)-4,7-dibe...)Show SMILES CS(=O)(=O)NCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNS(C)(=O)=O)C1=O Show InChI InChI=1S/C31H48N4O7S2/c1-43(39,40)32-19-11-5-13-21-34-27(23-25-15-7-3-8-16-25)29(36)30(37)28(24-26-17-9-4-10-18-26)35(31(34)38)22-14-6-12-20-33-44(2,41)42/h3-4,7-10,15-18,27-30,32-33,36-37H,5-6,11-14,19-24H2,1-2H3/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM117
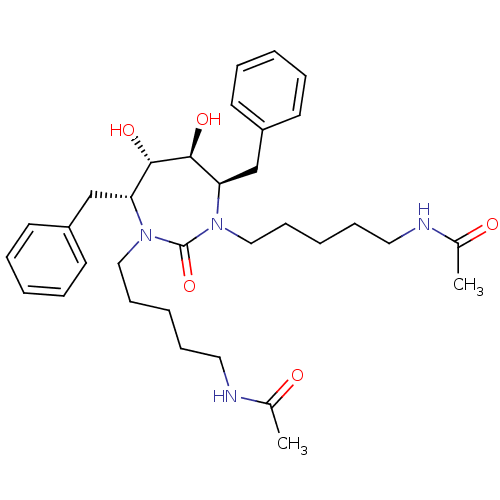
(DMPC Cyclic Urea 97 | N-{5-[(4R,5S,6S,7R)-4,7-dibe...)Show SMILES CC(=O)NCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCNC(C)=O)C1=O Show InChI InChI=1S/C33H48N4O5/c1-25(38)34-19-11-5-13-21-36-29(23-27-15-7-3-8-16-27)31(40)32(41)30(24-28-17-9-4-10-18-28)37(33(36)42)22-14-6-12-20-35-26(2)39/h3-4,7-10,15-18,29-32,40-41H,5-6,11-14,19-24H2,1-2H3,(H,34,38)(H,35,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM120
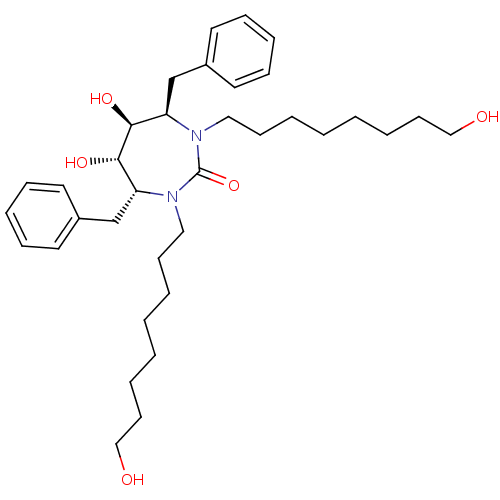
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(8...)Show SMILES OCCCCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCCCCO)C1=O Show InChI InChI=1S/C35H54N2O5/c38-25-17-7-3-1-5-15-23-36-31(27-29-19-11-9-12-20-29)33(40)34(41)32(28-30-21-13-10-14-22-30)37(35(36)42)24-16-6-2-4-8-18-26-39/h9-14,19-22,31-34,38-41H,1-8,15-18,23-28H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM128
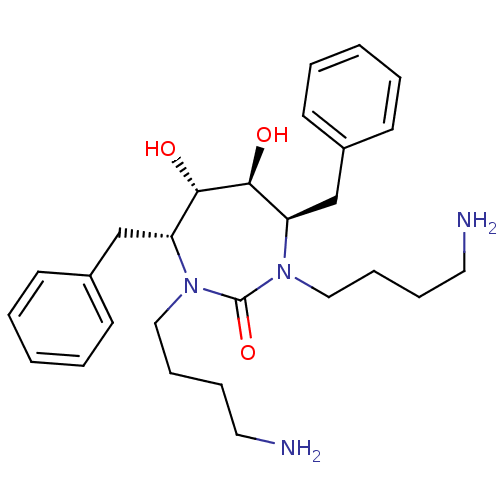
((4R,5S,6S,7R)-1,3-bis(4-aminobutyl)-4,7-dibenzyl-5...)Show SMILES NCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCN)C1=O Show InChI InChI=1S/C27H40N4O3/c28-15-7-9-17-30-23(19-21-11-3-1-4-12-21)25(32)26(33)24(20-22-13-5-2-6-14-22)31(27(30)34)18-10-8-16-29/h1-6,11-14,23-26,32-33H,7-10,15-20,28-29H2/t23-,24-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM127

((4R,5S,6S,7R)-1,3-bis(5-aminopentyl)-4,7-dibenzyl-...)Show SMILES NCCCCCN1[C@H](Cc2ccccc2)[C@H](O)[C@@H](O)[C@@H](Cc2ccccc2)N(CCCCCN)C1=O Show InChI InChI=1S/C29H44N4O3/c30-17-9-3-11-19-32-25(21-23-13-5-1-6-14-23)27(34)28(35)26(22-24-15-7-2-8-16-24)33(29(32)36)20-12-4-10-18-31/h1-2,5-8,13-16,25-28,34-35H,3-4,9-12,17-22,30-31H2/t25-,26-,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 1365-1370 (1997)
Article DOI: 10.1016/S0960-894X(97)00165-0
BindingDB Entry DOI: 10.7270/Q21G0M7B |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50433097
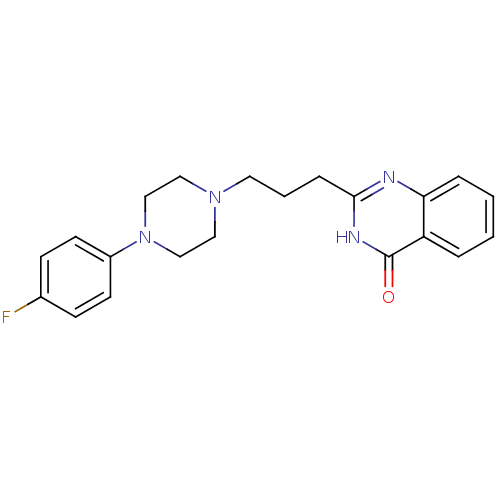
(CHEMBL2377258)Show SMILES Fc1ccc(cc1)N1CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C21H23FN4O/c22-16-7-9-17(10-8-16)26-14-12-25(13-15-26)11-3-6-20-23-19-5-2-1-4-18(19)21(27)24-20/h1-2,4-5,7-10H,3,6,11-15H2,(H,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50220856
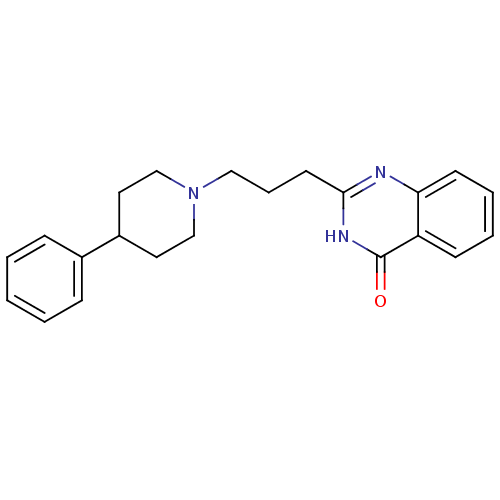
(2-(3-(4-phenylpiperidin-1-yl)propyl)quinazolin-4(3...)Show InChI InChI=1S/C22H25N3O/c26-22-19-9-4-5-10-20(19)23-21(24-22)11-6-14-25-15-12-18(13-16-25)17-7-2-1-3-8-17/h1-5,7-10,18H,6,11-16H2,(H,23,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50433098
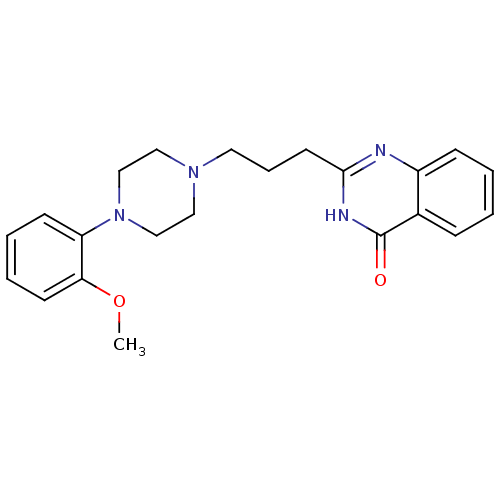
(CHEMBL2377257)Show SMILES COc1ccccc1N1CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C22H26N4O2/c1-28-20-10-5-4-9-19(20)26-15-13-25(14-16-26)12-6-11-21-23-18-8-3-2-7-17(18)22(27)24-21/h2-5,7-10H,6,11-16H2,1H3,(H,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50220864
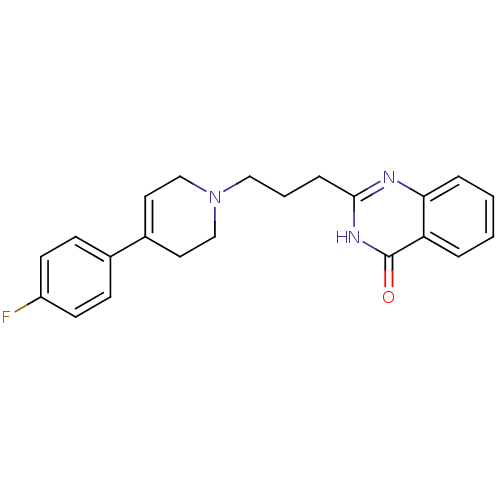
(2-(3-(4-(4-fluorophenyl)-5,6-dihydropyridin-1(2H)-...)Show SMILES Fc1ccc(cc1)C1=CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H22FN3O/c23-18-9-7-16(8-10-18)17-11-14-26(15-12-17)13-3-6-21-24-20-5-2-1-4-19(20)22(27)25-21/h1-2,4-5,7-11H,3,6,12-15H2,(H,24,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50433095
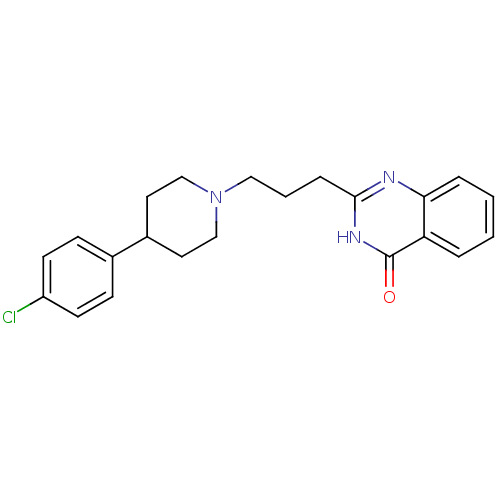
(CHEMBL2377260)Show SMILES Clc1ccc(cc1)C1CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C22H24ClN3O/c23-18-9-7-16(8-10-18)17-11-14-26(15-12-17)13-3-6-21-24-20-5-2-1-4-19(20)22(27)25-21/h1-2,4-5,7-10,17H,3,6,11-15H2,(H,24,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50433096
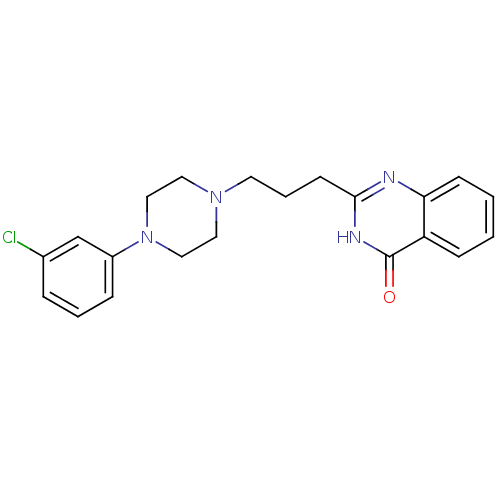
(CHEMBL2377259)Show SMILES Clc1cccc(c1)N1CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C21H23ClN4O/c22-16-5-3-6-17(15-16)26-13-11-25(12-14-26)10-4-9-20-23-19-8-2-1-7-18(19)21(27)24-20/h1-3,5-8,15H,4,9-14H2,(H,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311577
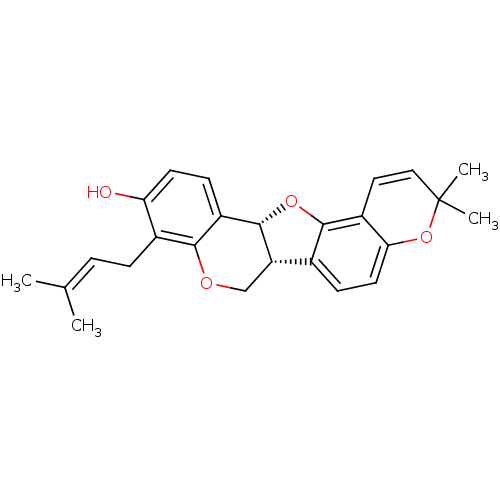
(CHEMBL561967 | Erybreadin B | erybraedin B)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6@@H]-3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)-[#6@@H]-3-[#6]-[#8]-c12 |r,c:22| Show InChI InChI=1S/C25H26O4/c1-14(2)5-6-16-20(26)9-7-18-22(16)27-13-19-15-8-10-21-17(23(15)28-24(18)19)11-12-25(3,4)29-21/h5,7-12,19,24,26H,6,13H2,1-4H3/t19-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
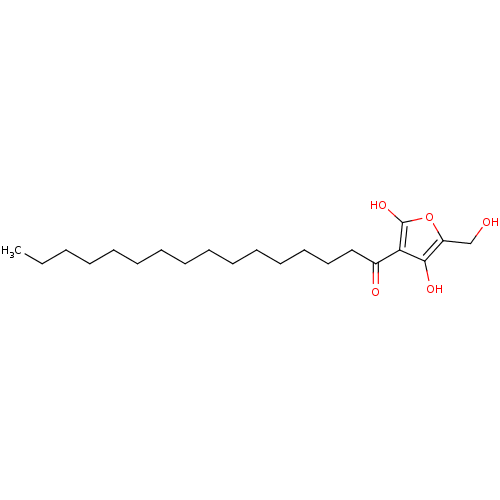
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311580
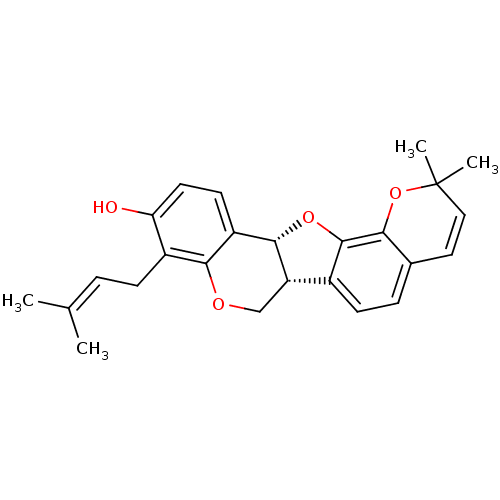
(CHEMBL1087148 | Erybreadin D)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6@@H]-3-[#8]-c4c(ccc5-[#6]=[#6]C([#6])([#6])[#8]-c45)-[#6@@H]-3-[#6]-[#8]-c12 |r,c:18| Show InChI InChI=1S/C25H26O4/c1-14(2)5-7-17-20(26)10-9-18-22(17)27-13-19-16-8-6-15-11-12-25(3,4)29-21(15)24(16)28-23(18)19/h5-6,8-12,19,23,26H,7,13H2,1-4H3/t19-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311582
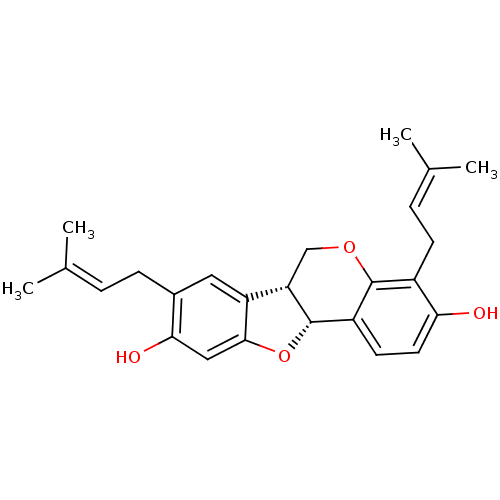
(CHEMBL1086765 | Erybreadin C)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#6]-[#8]-c4c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])ccc4-[#6@@H]-3-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C25H28O4/c1-14(2)5-7-16-11-19-20-13-28-24-17(8-6-15(3)4)21(26)10-9-18(24)25(20)29-23(19)12-22(16)27/h5-6,9-12,20,25-27H,7-8,13H2,1-4H3/t20-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50317432
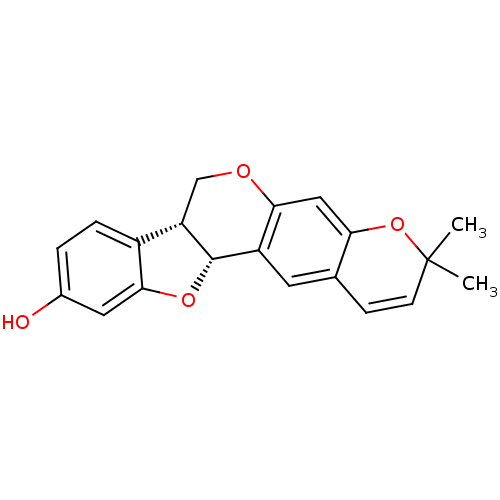
(CHEMBL1098728 | NEORAUTENOL)Show SMILES CC1(C)Oc2cc3OC[C@@H]4[C@@H](Oc5cc(O)ccc45)c3cc2C=C1 |r,c:26| Show InChI InChI=1S/C20H18O4/c1-20(2)6-5-11-7-14-17(9-16(11)24-20)22-10-15-13-4-3-12(21)8-18(13)23-19(14)15/h3-9,15,19,21H,10H2,1-2H3/t15-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311579
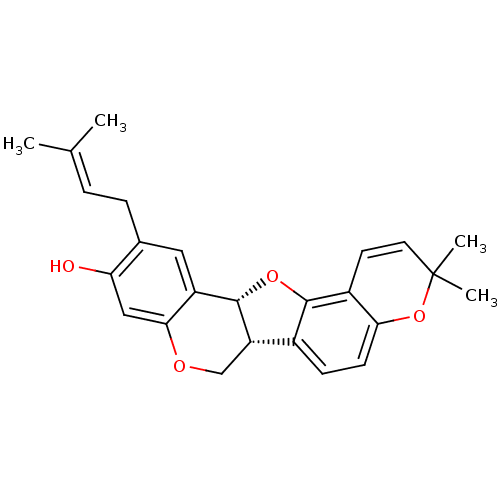
(CHEMBL551155 | folitenol)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-[#6@@H]-3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)-[#6@@H]-3-[#6]-[#8]-c2cc1-[#8] |r,c:19| Show InChI InChI=1S/C25H26O4/c1-14(2)5-6-15-11-18-22(12-20(15)26)27-13-19-16-7-8-21-17(23(16)28-24(18)19)9-10-25(3,4)29-21/h5,7-12,19,24,26H,6,13H2,1-4H3/t19-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311581
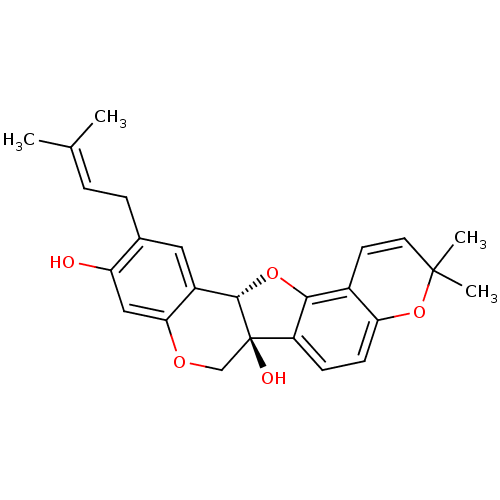
(CHEMBL1086764 | erysubin E)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc2-[#6@@H]3-[#8]-c4c(ccc5-[#8]C([#6])([#6])[#6]=[#6]-c45)[C@]3([#8])[#6]-[#8]-c2cc1-[#8] |r,c:19| Show InChI InChI=1S/C25H26O5/c1-14(2)5-6-15-11-17-21(12-19(15)26)28-13-25(27)18-7-8-20-16(22(18)29-23(17)25)9-10-24(3,4)30-20/h5,7-12,23,26-27H,6,13H2,1-4H3/t23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50433099
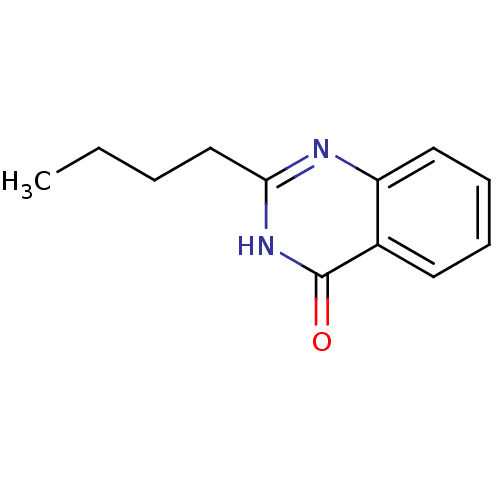
(CHEMBL2377256)Show InChI InChI=1S/C12H14N2O/c1-2-3-8-11-13-10-7-5-4-6-9(10)12(15)14-11/h4-7H,2-3,8H2,1H3,(H,13,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan [corrected] University
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 (unknown origin) after 15 mins by fluorometric analysis |
Bioorg Med Chem Lett 23: 2642-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.094
BindingDB Entry DOI: 10.7270/Q2WH2RCN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311572
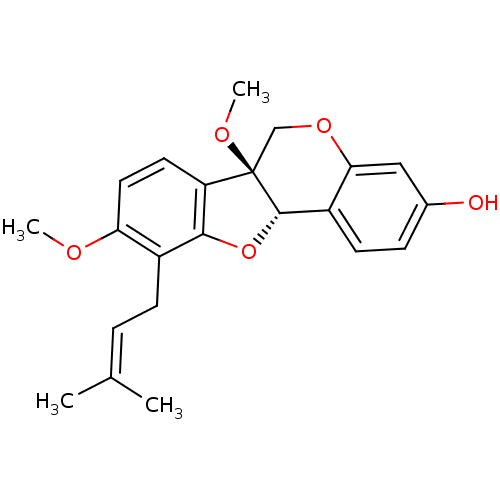
(CHEMBL1076430 | Erythribyssin A)Show SMILES [#6]-[#8]-c1ccc2c(-[#8]-[#6@H]3-c4ccc(-[#8])cc4-[#8]-[#6][C@@]23[#8]-[#6])c1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H24O5/c1-13(2)5-7-15-18(24-3)10-9-17-20(15)27-21-16-8-6-14(23)11-19(16)26-12-22(17,21)25-4/h5-6,8-11,21,23H,7,12H2,1-4H3/t21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311578

(3,9-Dihydroxy-4-prenyl-[6aR;11aR]pterocarpan | CHE...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6@@H]-3-[#8]-c4cc(-[#8])ccc4-[#6@@H]-3-[#6]-[#8]-c12 |r| Show InChI InChI=1S/C20H20O4/c1-11(2)3-5-14-17(22)8-7-15-19(14)23-10-16-13-6-4-12(21)9-18(13)24-20(15)16/h3-4,6-9,16,20-22H,5,10H2,1-2H3/t16-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311586
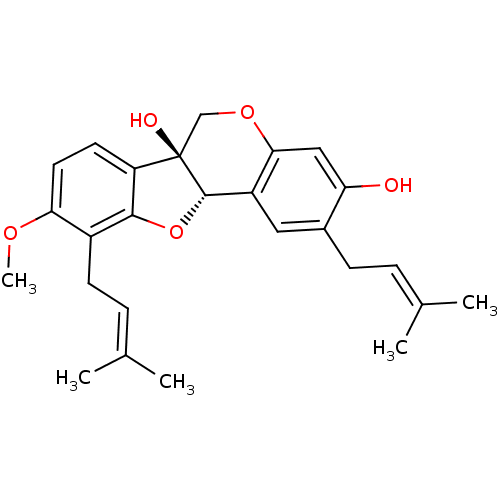
(CHEMBL1088462 | erystagallin A)Show SMILES [#6]-[#8]-c1ccc2c(-[#8]-[#6@H]3-c4cc(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc4-[#8]-[#6][C@@]23[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C26H30O5/c1-15(2)6-8-17-12-19-23(13-21(17)27)30-14-26(28)20-10-11-22(29-5)18(9-7-16(3)4)24(20)31-25(19)26/h6-7,10-13,25,27-28H,8-9,14H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311583
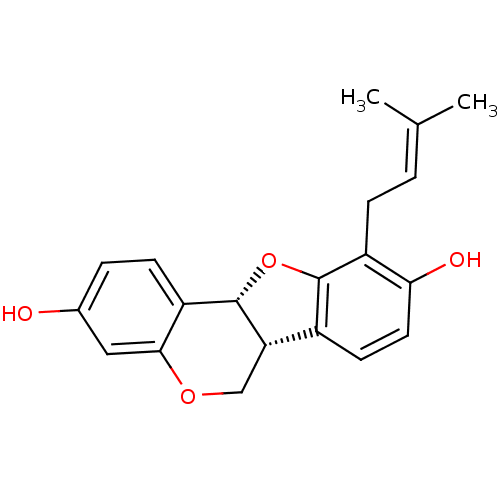
(Abyssinone II | CHEMBL508534 | phaseolidin | phase...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c2-[#8]-[#6@@H]-3-[#6@@H](-[#6]-[#8]-c4cc(-[#8])ccc-34)-c2ccc1-[#8] |r| Show InChI InChI=1S/C20H20O4/c1-11(2)3-5-14-17(22)8-7-13-16-10-23-18-9-12(21)4-6-15(18)20(16)24-19(13)14/h3-4,6-9,16,20-22H,5,10H2,1-2H3/t16-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311575
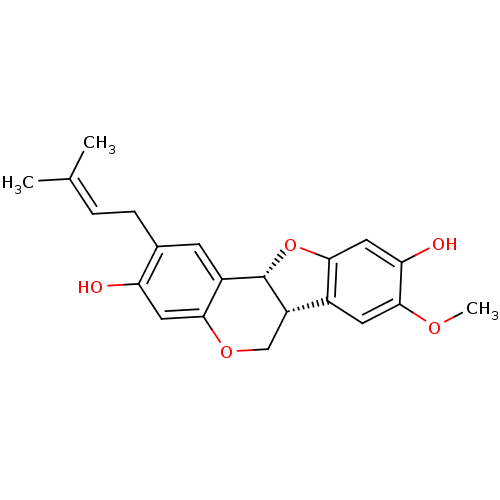
(CHEMBL1079408 | Eryvarin K)Show SMILES [#6]-[#8]-c1cc2-[#6@@H]-3-[#6]-[#8]-c4cc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])cc4-[#6@@H]-3-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C21H22O5/c1-11(2)4-5-12-6-14-18(8-16(12)22)25-10-15-13-7-20(24-3)17(23)9-19(13)26-21(14)15/h4,6-9,15,21-23H,5,10H2,1-3H3/t15-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311574

(CHEMBL1079407 | Erythribyssin C)Show SMILES [#6]-[#8]-c1cc2-[#6@@H]-3-[#6]-[#8]-c4cc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])cc4-[#6@@H]-3-[#8]-c2cc1-[#8] |r| Show InChI InChI=1S/C22H24O5/c1-12(2)5-6-13-7-15-19(10-18(13)24-3)26-11-16-14-8-21(25-4)17(23)9-20(14)27-22(15)16/h5,7-10,16,22-23H,6,11H2,1-4H3/t16-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311573

(CHEMBL1079406 | Erythribyssin B)Show SMILES Oc1ccc2[C@@H]3Oc4c(ccc(O)c4C=O)[C@@H]3COc2c1 |r| Show InChI InChI=1S/C16H12O5/c17-6-11-13(19)4-3-9-12-7-20-14-5-8(18)1-2-10(14)16(12)21-15(9)11/h1-6,12,16,18-19H,7H2/t12-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B assessed as p-nitrophenol production |
Bioorg Med Chem Lett 19: 6745-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.108
BindingDB Entry DOI: 10.7270/Q24B328V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data