

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100528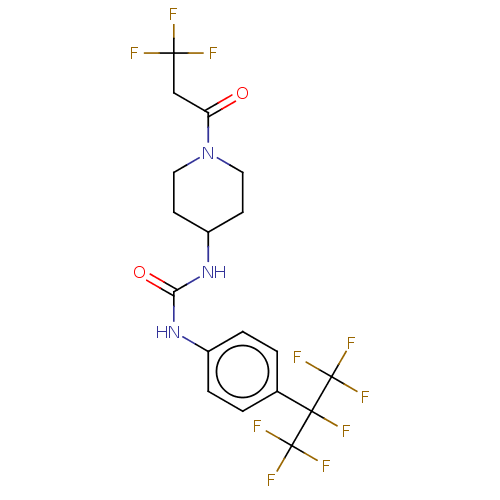 (CHEMBL3327081) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100535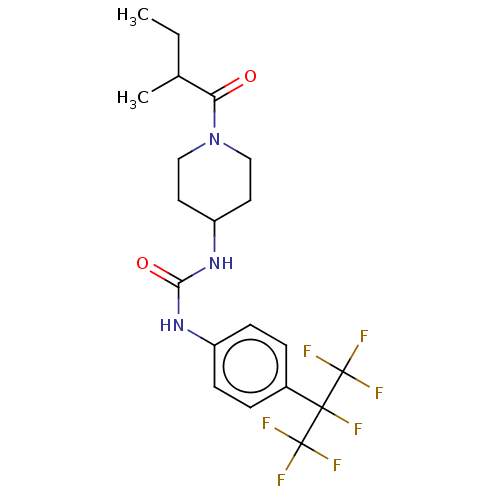 (CHEMBL3327073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409020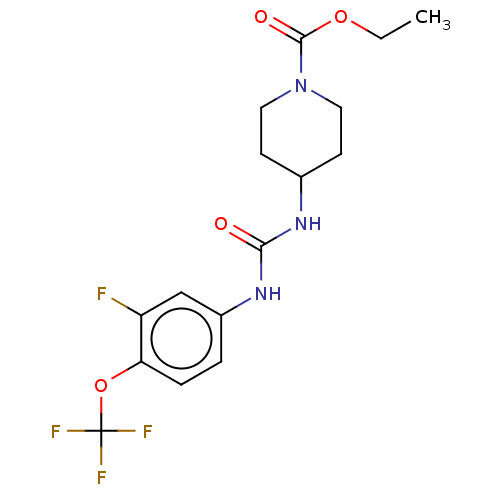 (US10377744, Compound No. 40 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005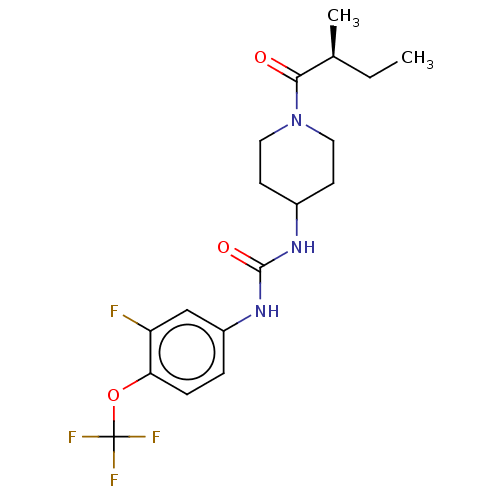 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409009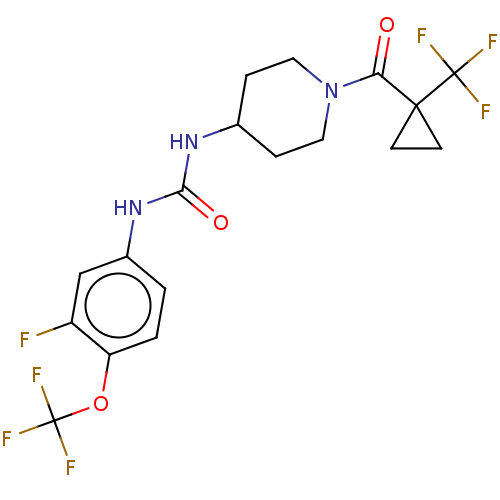 (US10377744, Compound No. 30 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409005 (US10377744, Compound No. 26 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409007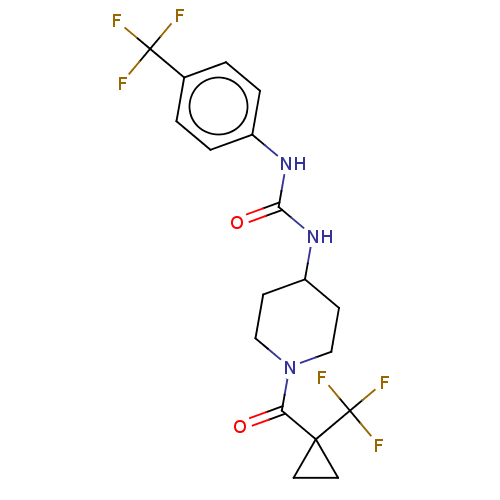 (US10377744, Compound No. 28 | US11723929, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409009 (US10377744, Compound No. 30 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409020 (US10377744, Compound No. 40 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409030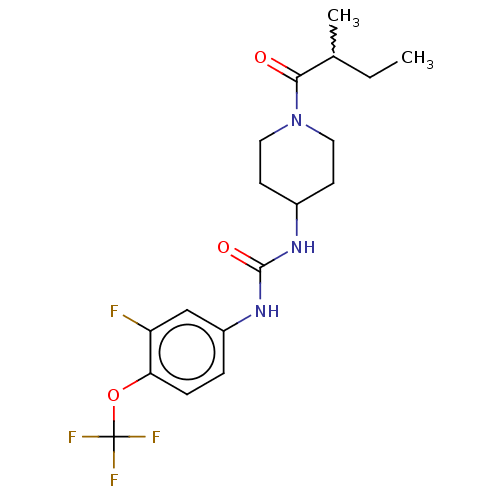 (US10377744, Compound No. 51 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409016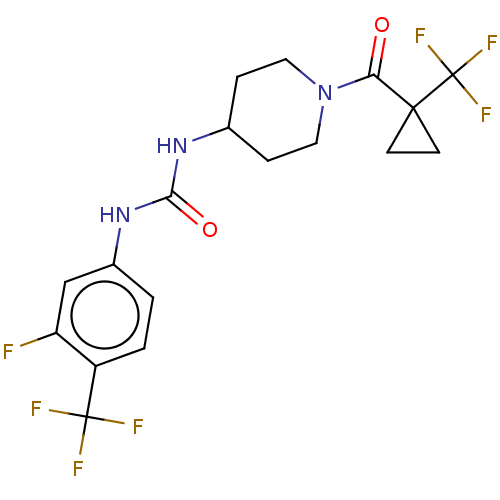 (US10377744, Compound No. 36 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409016 (US10377744, Compound No. 36 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111741 (CHEMBL19666 | N-(5-Carbamimidoyl-thiophen-2-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111728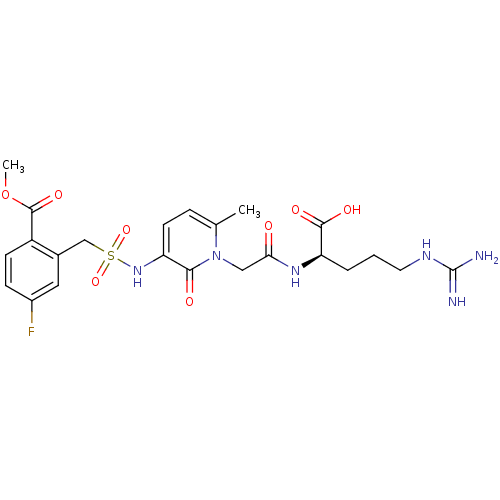 (4-Fluoro-2-({1-[((R)-1-formyl-4-guanidino-butylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111729 (CHEMBL277695 | N-(5-Carbamimidoyl-thiophen-2-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111730 (2-[3-(2-Fluoro-phenylmethanesulfonylamino)-6-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003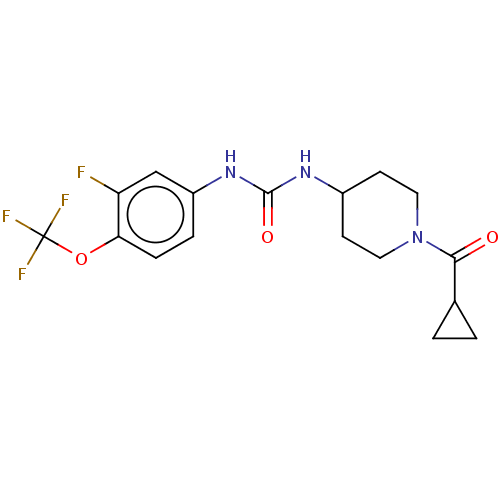 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409003 (US10377744, Compound No. 24 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100521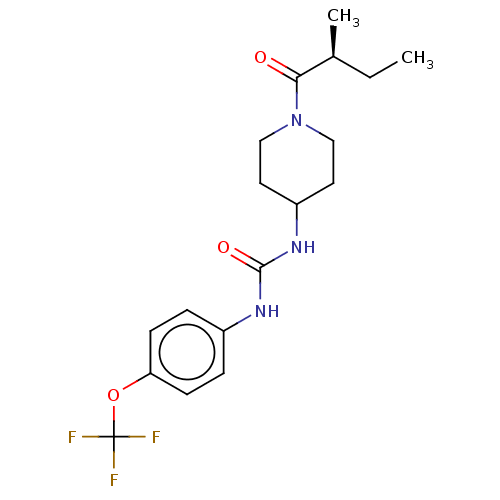 (CHEMBL3327078 | US10377744, Compound No. 2696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100521 (CHEMBL3327078 | US10377744, Compound No. 2696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519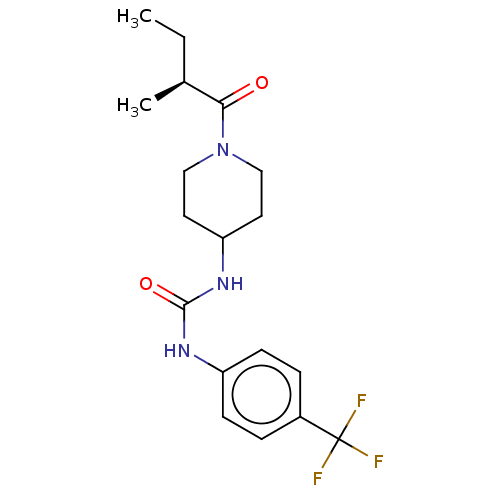 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408992 (US10377744, Compound No. 13 | US10377744, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50554114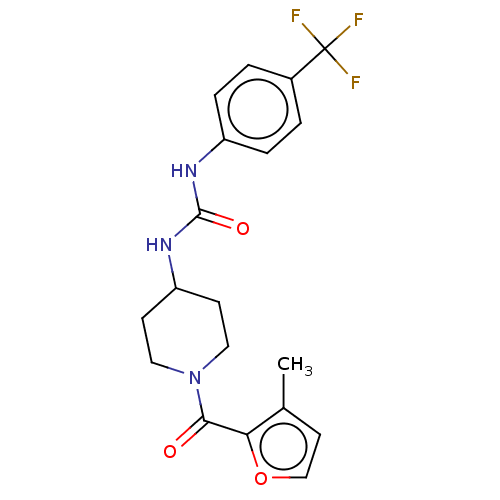 (CHEMBL4781745) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100531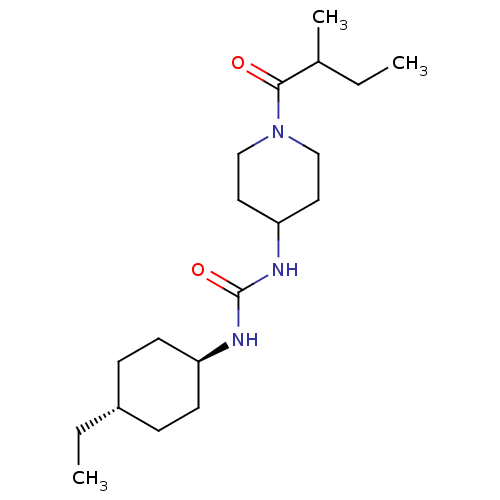 (CHEMBL3325465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408986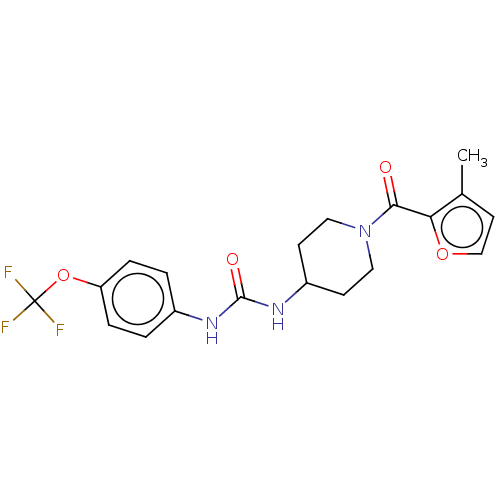 (US10377744, Compound No. 7 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408986 (US10377744, Compound No. 7 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520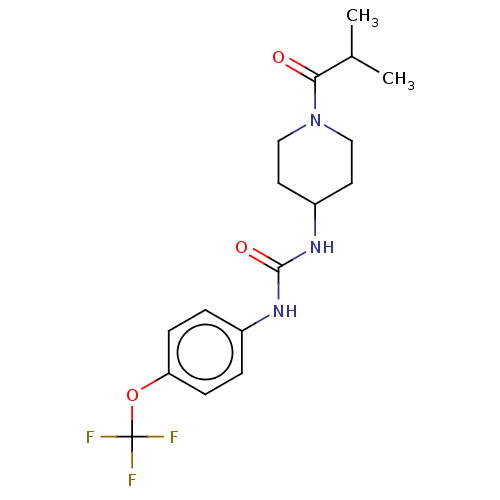 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998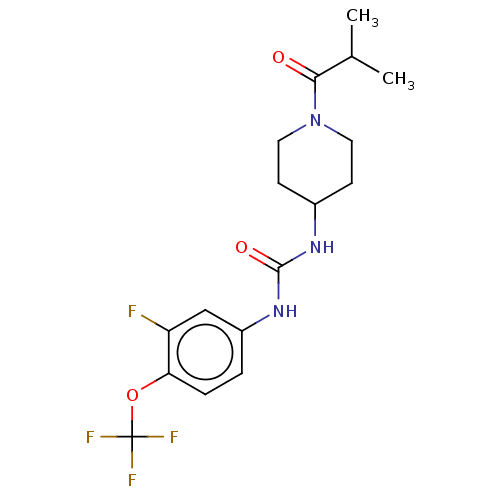 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408998 (US10377744, Compound No. 19 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408982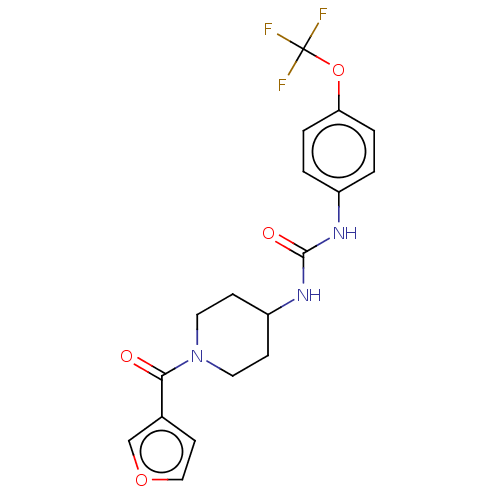 (US10377744, Compound No. 3 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM408982 (US10377744, Compound No. 3 | US11123311, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100534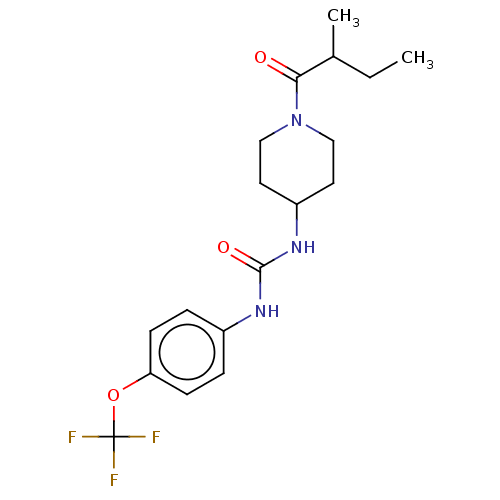 (CHEMBL3327074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014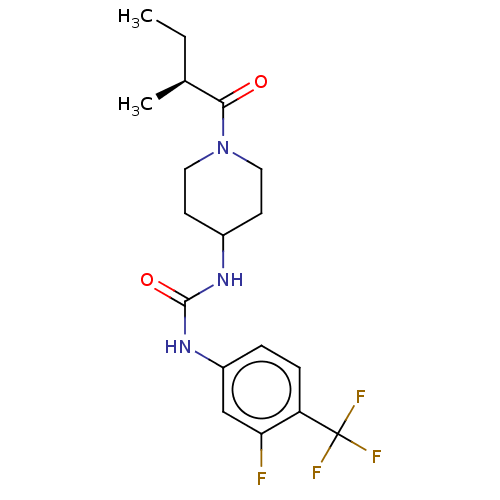 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111724 (CHEMBL19731 | N-(4-Carbamimidoyl-benzyl)-2-(6-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to purified recombinant human sEH by FRET-displacement assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01886 BindingDB Entry DOI: 10.7270/Q22V2KSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409014 (US10377744, Compound No. 34 | US10377744, Syn34 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100541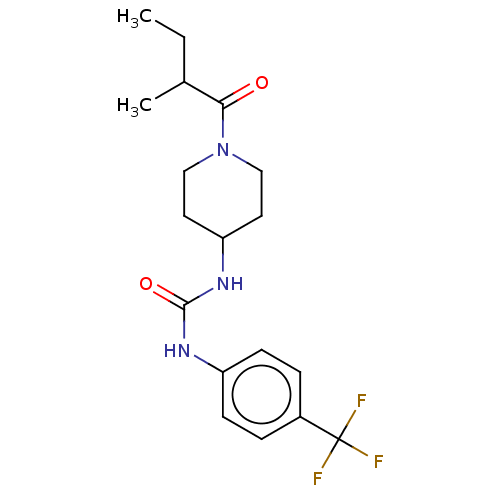 (CHEMBL3327066) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409012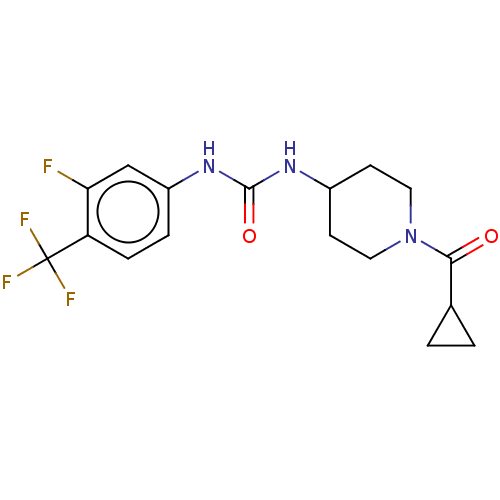 (US10377744, Compound No. 32 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409021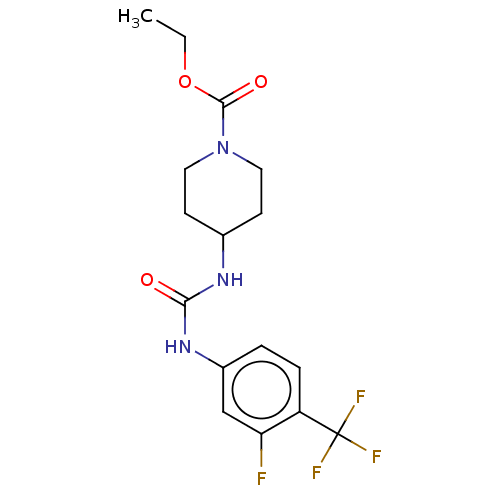 (US10377744, Compound No. 41 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115735 BindingDB Entry DOI: 10.7270/Q2SF30T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409021 (US10377744, Compound No. 41 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM409012 (US10377744, Compound No. 32 | US11123311, Compound...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC US Patent | Assay Description FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... | US Patent US10377744 (2019) BindingDB Entry DOI: 10.7270/Q2N3009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100539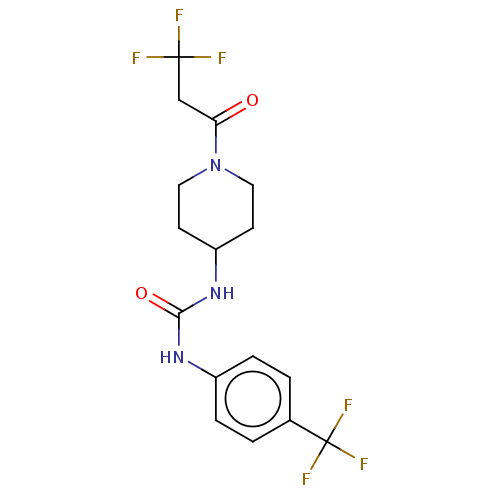 (CHEMBL3327069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 806 total ) | Next | Last >> |