 Found 420 hits with Last Name = 'levesque' and Initial = 'pc'
Found 420 hits with Last Name = 'levesque' and Initial = 'pc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017021
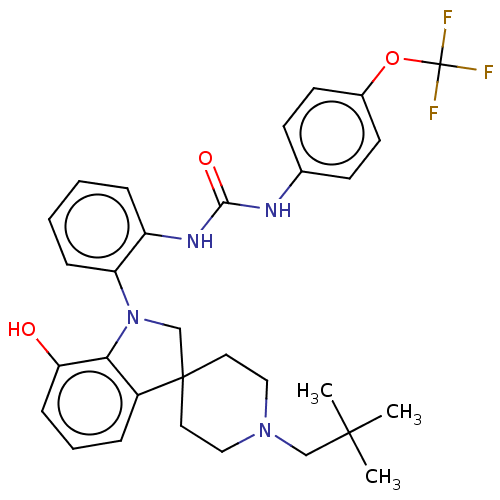
(CHEMBL3287047 | US9428504, 1)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2cccc3O)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H35F3N4O3/c1-29(2,3)19-37-17-15-30(16-18-37)20-38(27-23(30)7-6-10-26(27)39)25-9-5-4-8-24(25)36-28(40)35-21-11-13-22(14-12-21)41-31(32,33)34/h4-14,39H,15-20H2,1-3H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to P2Y1 receptor in human platelets |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470
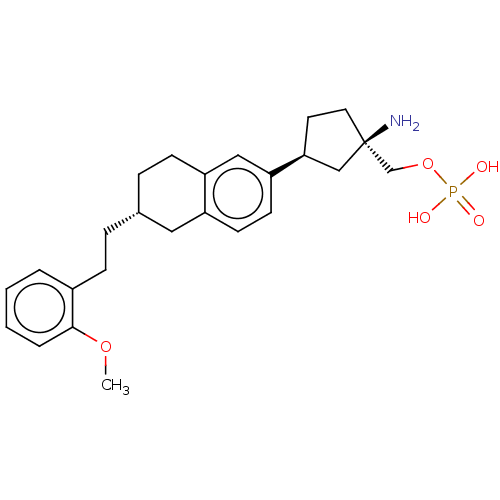
(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258466
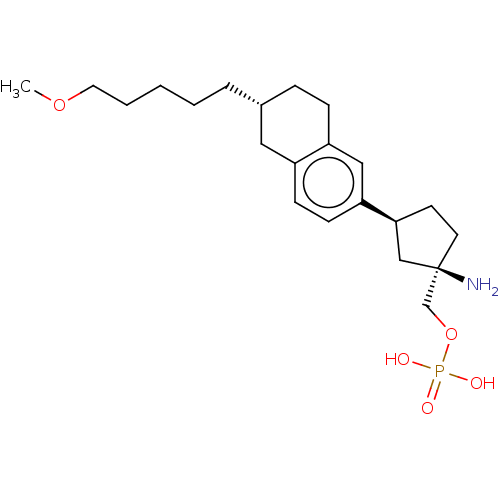
(US9522888, 689)Show SMILES COCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO5P/c1-27-12-4-2-3-5-17-6-7-19-14-20(9-8-18(19)13-17)21-10-11-22(23,15-21)16-28-29(24,25)26/h8-9,14,17,21H,2-7,10-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017084

(CHEMBL3287050 | US9428504, 61)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2csc(Cl)n2)CC1 Show InChI InChI=1S/C27H31Cl2N5O2S/c1-26(2,3)15-33-12-10-27(11-13-33)16-34(23-20(35)9-8-17(28)22(23)27)19-7-5-4-6-18(19)30-25(36)32-21-14-37-24(29)31-21/h4-9,14,35H,10-13,15-16H2,1-3H3,(H2,30,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50562873
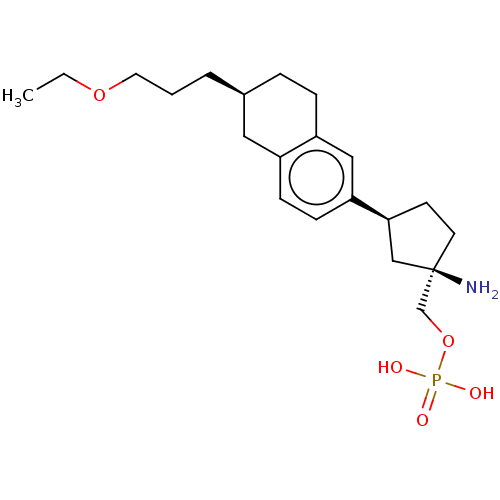
(CHEMBL4786296)Show SMILES CCOCCC[C@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017089
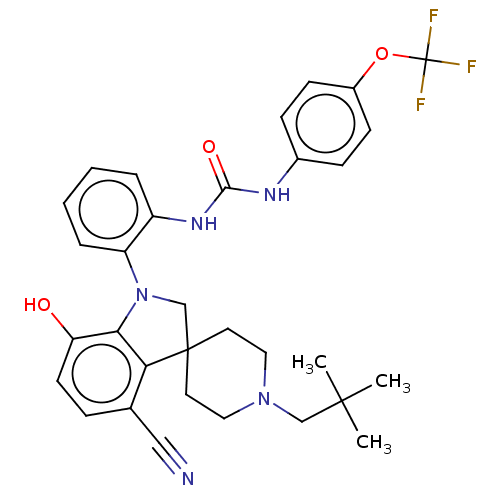
(CHEMBL3287043 | US9428504, 8)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(ccc3O)C#N)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C32H34F3N5O3/c1-30(2,3)19-39-16-14-31(15-17-39)20-40(28-26(41)13-8-21(18-36)27(28)31)25-7-5-4-6-24(25)38-29(42)37-22-9-11-23(12-10-22)43-32(33,34)35/h4-13,41H,14-17,19-20H2,1-3H3,(H2,37,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017090

(CHEMBL3287044 | US9428504, 59)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H34ClF3N4O3/c1-29(2,3)18-38-16-14-30(15-17-38)19-39(27-25(40)13-12-22(32)26(27)30)24-7-5-4-6-23(24)37-28(41)36-20-8-10-21(11-9-20)42-31(33,34)35/h4-13,40H,14-19H2,1-3H3,(H2,36,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017083

(CHEMBL3287049 | US9428504, 39)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2nc3ccc(F)cc3s2)CC1 Show InChI InChI=1S/C31H33ClFN5O2S/c1-30(2,3)17-37-14-12-31(13-15-37)18-38(27-24(39)11-9-20(32)26(27)31)23-7-5-4-6-21(23)34-28(40)36-29-35-22-10-8-19(33)16-25(22)41-29/h4-11,16,39H,12-15,17-18H2,1-3H3,(H2,34,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017085
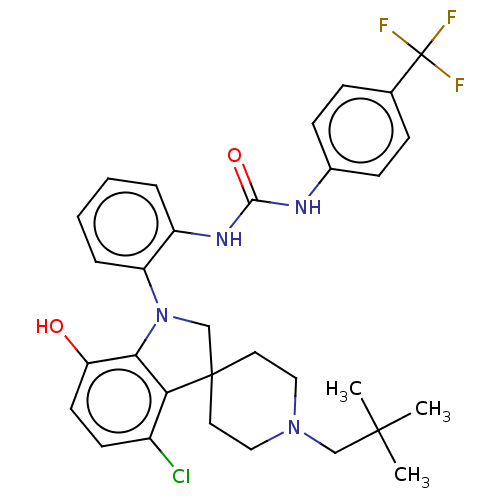
(CHEMBL3287051 | US9428504, 60)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2ccc(cc2)C(F)(F)F)CC1 Show InChI InChI=1S/C31H34ClF3N4O2/c1-29(2,3)18-38-16-14-30(15-17-38)19-39(27-25(40)13-12-22(32)26(27)30)24-7-5-4-6-23(24)37-28(41)36-21-10-8-20(9-11-21)31(33,34)35/h4-13,40H,14-19H2,1-3H3,(H2,36,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718
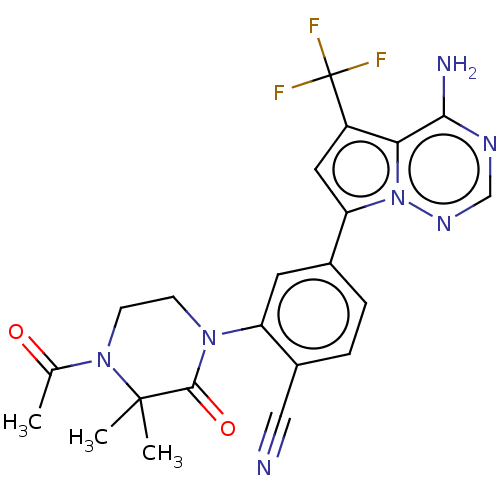
(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532543
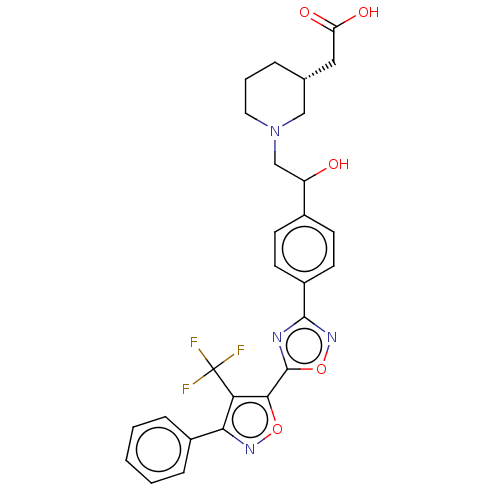
(CHEMBL4474984)Show SMILES OC(CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532543

(CHEMBL4474984)Show SMILES OC(CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50015276
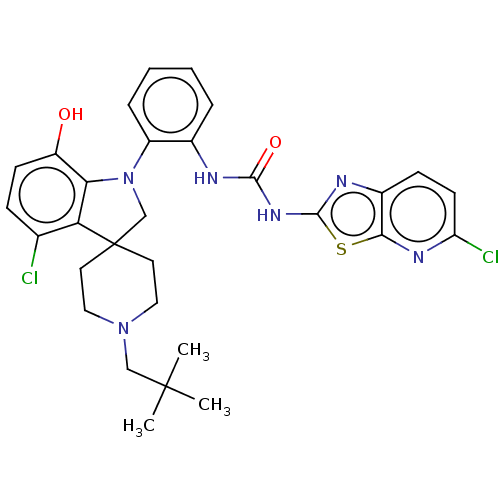
(CHEMBL3263056 | US9428504, 166 | US9428504, 167)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2nc3ccc(Cl)nc3s2)CC1 Show InChI InChI=1S/C30H32Cl2N6O2S/c1-29(2,3)16-37-14-12-30(13-15-37)17-38(25-22(39)10-8-18(31)24(25)30)21-7-5-4-6-19(21)33-27(40)36-28-34-20-9-11-23(32)35-26(20)41-28/h4-11,39H,12-17H2,1-3H3,(H2,33,34,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017088

(CHEMBL3287042 | US9428504, 89)Show SMILES CN(C)S(=O)(=O)c1ccc(O)c2N(CC3(CCN(CC(C)(C)C)CC3)c12)c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C33H40F3N5O5S/c1-31(2,3)20-40-18-16-32(17-19-40)21-41(29-26(42)14-15-27(28(29)32)47(44,45)39(4)5)25-9-7-6-8-24(25)38-30(43)37-22-10-12-23(13-11-22)46-33(34,35)36/h6-15,42H,16-21H2,1-5H3,(H2,37,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532539
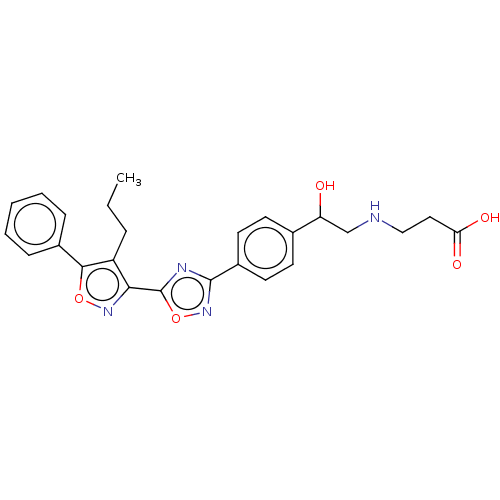
(CHEMBL4569675)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CNCCC(O)=O Show InChI InChI=1S/C25H26N4O5/c1-2-6-19-22(28-33-23(19)17-7-4-3-5-8-17)25-27-24(29-34-25)18-11-9-16(10-12-18)20(30)15-26-14-13-21(31)32/h3-5,7-12,20,26,30H,2,6,13-15H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532539

(CHEMBL4569675)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CNCCC(O)=O Show InChI InChI=1S/C25H26N4O5/c1-2-6-19-22(28-33-23(19)17-7-4-3-5-8-17)25-27-24(29-34-25)18-11-9-16(10-12-18)20(30)15-26-14-13-21(31)32/h3-5,7-12,20,26,30H,2,6,13-15H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017087
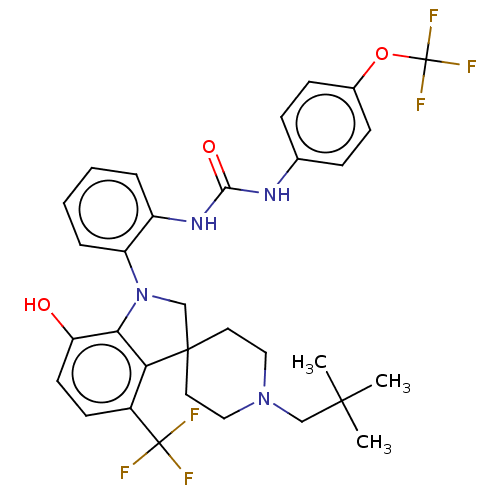
(CHEMBL3287041 | US9428504, 10)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(ccc3O)C(F)(F)F)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C32H34F6N4O3/c1-29(2,3)18-41-16-14-30(15-17-41)19-42(27-25(43)13-12-22(26(27)30)31(33,34)35)24-7-5-4-6-23(24)40-28(44)39-20-8-10-21(11-9-20)45-32(36,37)38/h4-13,43H,14-19H2,1-3H3,(H2,39,40,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017086

(CHEMBL3287052 | US9428504, 79)Show SMILES COC(=O)c1ccc(O)c2N(CC3(CCN(CC(C)(C)C)CC3)c12)c1ccccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C33H37F3N4O5/c1-31(2,3)19-39-17-15-32(16-18-39)20-40(28-26(41)14-13-23(27(28)32)29(42)44-4)25-8-6-5-7-24(25)38-30(43)37-21-9-11-22(12-10-21)45-33(34,35)36/h5-14,41H,15-20H2,1-4H3,(H2,37,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017075
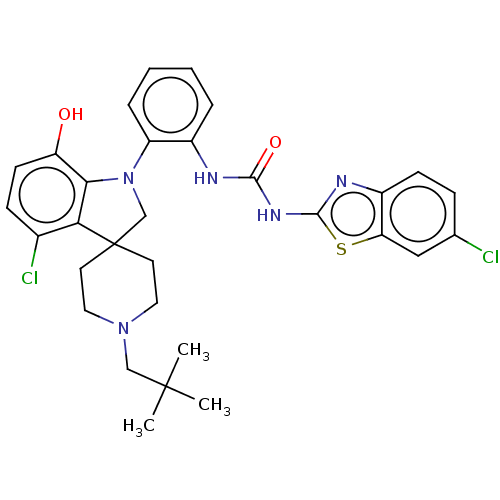
(CHEMBL3287040 | US9428504, 38)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(Cl)ccc3O)c2ccccc2NC(=O)Nc2nc3ccc(Cl)cc3s2)CC1 Show InChI InChI=1S/C31H33Cl2N5O2S/c1-30(2,3)17-37-14-12-31(13-15-37)18-38(27-24(39)11-9-20(33)26(27)31)23-7-5-4-6-21(23)34-28(40)36-29-35-22-10-8-19(32)16-25(22)41-29/h4-11,16,39H,12-15,17-18H2,1-3H3,(H2,34,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532532

(CHEMBL4457691)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532532

(CHEMBL4457691)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C29H32N4O5/c1-2-7-23-26(31-37-27(23)21-9-4-3-5-10-21)29-30-28(32-38-29)22-13-11-20(12-14-22)24(34)18-33-15-6-8-19(17-33)16-25(35)36/h3-5,9-14,19,24,34H,2,6-8,15-18H2,1H3,(H,35,36)/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017021

(CHEMBL3287047 | US9428504, 1)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2cccc3O)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H35F3N4O3/c1-29(2,3)19-37-17-15-30(16-18-37)20-38(27-23(30)7-6-10-26(27)39)25-9-5-4-8-24(25)36-28(40)35-21-11-13-22(14-12-21)41-31(32,33)34/h4-14,39H,15-20H2,1-3H3,(H2,35,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50017091
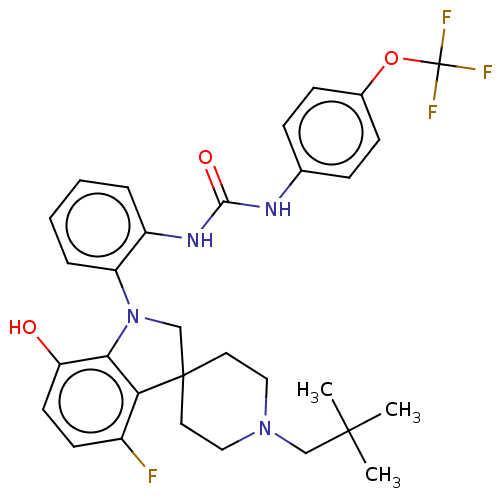
(CHEMBL3287045 | US9428504, 6)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(F)ccc3O)c2ccccc2NC(=O)Nc2ccc(OC(F)(F)F)cc2)CC1 Show InChI InChI=1S/C31H34F4N4O3/c1-29(2,3)18-38-16-14-30(15-17-38)19-39(27-25(40)13-12-22(32)26(27)30)24-7-5-4-6-23(24)37-28(41)36-20-8-10-21(11-9-20)42-31(33,34)35/h4-13,40H,14-19H2,1-3H3,(H2,36,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP |
Bioorg Med Chem Lett 24: 1294-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.066
BindingDB Entry DOI: 10.7270/Q20G3MQF |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532542
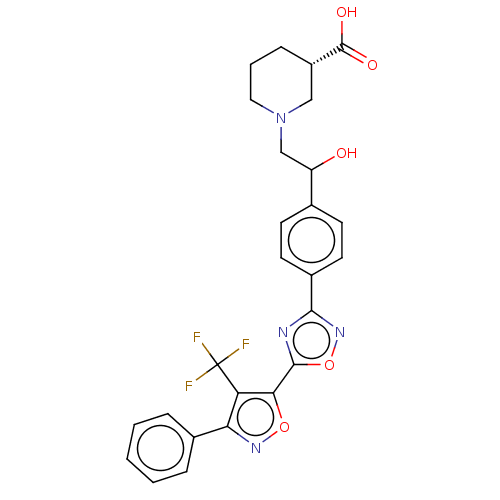
(CHEMBL4461520)Show SMILES OC(CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532542

(CHEMBL4461520)Show SMILES OC(CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433
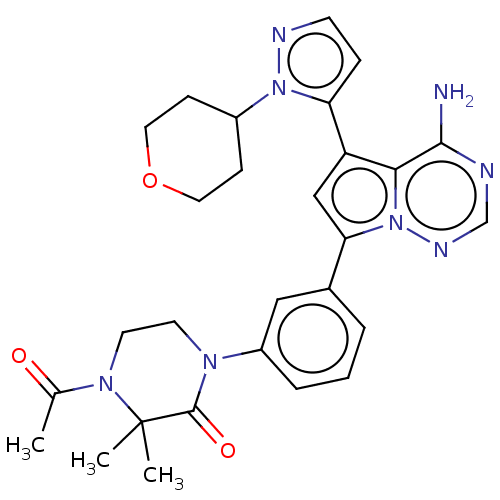
(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532534

(CHEMBL4469843)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@H]1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532534

(CHEMBL4469843)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN[C@H]1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-21-24(31-36-25(21)18-8-4-3-5-9-18)27-30-26(32-37-27)19-14-12-17(13-15-19)23(33)16-29-22-11-6-10-20(22)28(34)35/h3-5,8-9,12-15,20,22-23,29,33H,2,6-7,10-11,16H2,1H3,(H,34,35)/t20-,22+,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532526
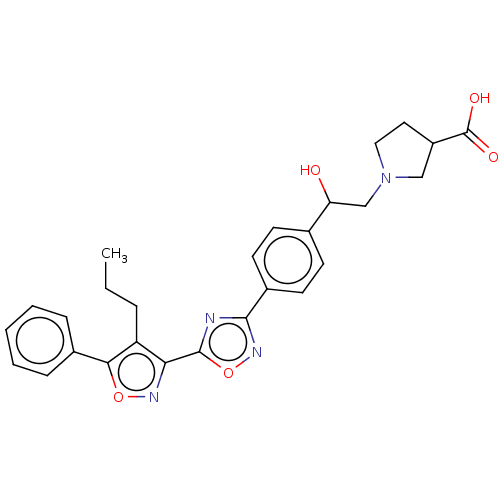
(CHEMBL4440968)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC(C1)C(O)=O Show InChI InChI=1S/C27H28N4O5/c1-2-6-21-23(29-35-24(21)18-7-4-3-5-8-18)26-28-25(30-36-26)19-11-9-17(10-12-19)22(32)16-31-14-13-20(15-31)27(33)34/h3-5,7-12,20,22,32H,2,6,13-16H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532526

(CHEMBL4440968)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC(C1)C(O)=O Show InChI InChI=1S/C27H28N4O5/c1-2-6-21-23(29-35-24(21)18-7-4-3-5-8-18)26-28-25(30-36-26)19-11-9-17(10-12-19)22(32)16-31-14-13-20(15-31)27(33)34/h3-5,7-12,20,22,32H,2,6,13-16H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472
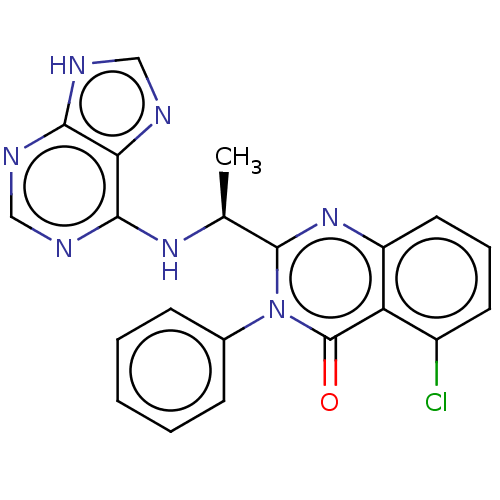
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239736
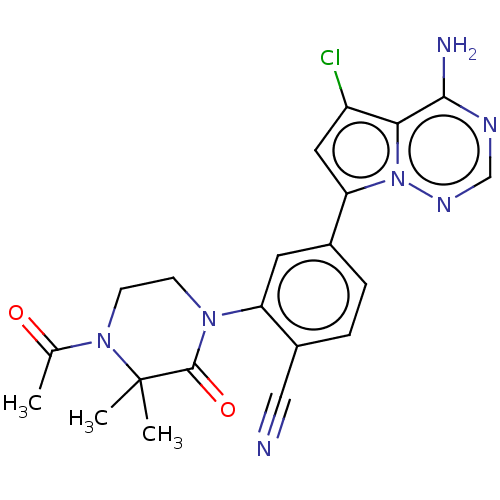
(CHEMBL4074315 | US10214537, Example 637)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H20ClN7O2/c1-12(30)28-7-6-27(20(31)21(28,2)3)16-8-13(4-5-14(16)10-23)17-9-15(22)18-19(24)25-11-26-29(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532538
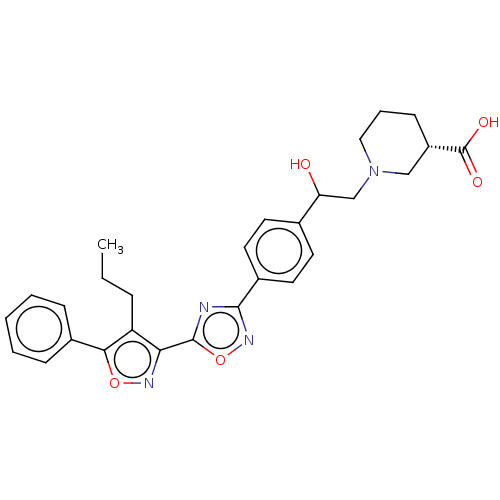
(CHEMBL4587424)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-22-24(30-36-25(22)19-8-4-3-5-9-19)27-29-26(31-37-27)20-13-11-18(12-14-20)23(33)17-32-15-6-10-21(16-32)28(34)35/h3-5,8-9,11-14,21,23,33H,2,6-7,10,15-17H2,1H3,(H,34,35)/t21-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532538

(CHEMBL4587424)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN1CCC[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C28H30N4O5/c1-2-7-22-24(30-36-25(22)19-8-4-3-5-9-19)27-29-26(31-37-27)20-13-11-18(12-14-20)23(33)17-32-15-6-10-21(16-32)28(34)35/h3-5,8-9,11-14,21,23,33H,2,6-7,10,15-17H2,1H3,(H,34,35)/t21-,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239744
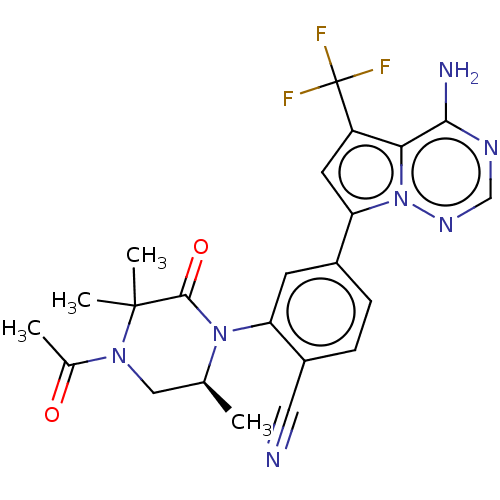
(CHEMBL4071965 | US10214537, Example 643)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C23H22F3N7O2/c1-12-10-31(13(2)34)22(3,4)21(35)32(12)17-7-14(5-6-15(17)9-27)18-8-16(23(24,25)26)19-20(28)29-11-30-33(18)19/h5-8,11-12H,10H2,1-4H3,(H2,28,29,30)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239752
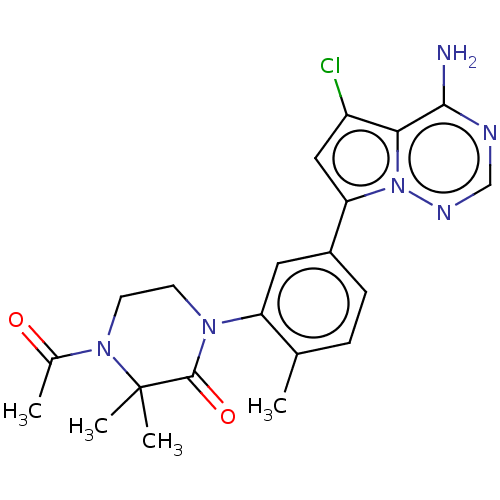
(CHEMBL4067315 | US10214537, Example 585)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H23ClN6O2/c1-12-5-6-14(17-10-15(22)18-19(23)24-11-25-28(17)18)9-16(12)26-7-8-27(13(2)29)21(3,4)20(26)30/h5-6,9-11H,7-8H2,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239741
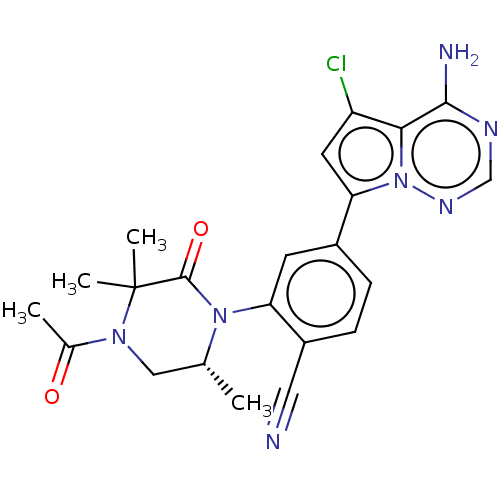
(CHEMBL4095752)Show SMILES C[C@@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H22ClN7O2/c1-12-10-28(13(2)31)22(3,4)21(32)29(12)17-7-14(5-6-15(17)9-24)18-8-16(23)19-20(25)26-11-27-30(18)19/h5-8,11-12H,10H2,1-4H3,(H2,25,26,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239746
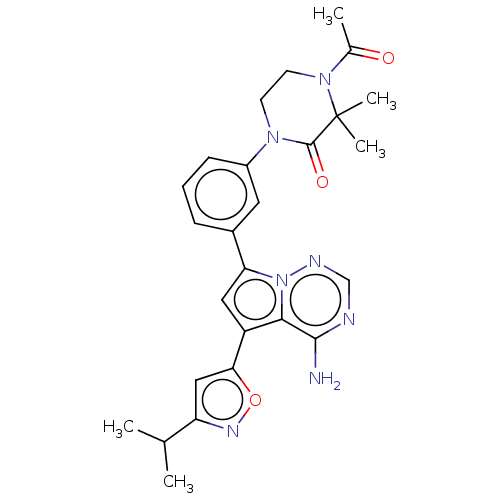
(CHEMBL4094693)Show SMILES CC(C)c1cc(on1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C26H29N7O3/c1-15(2)20-13-22(36-30-20)19-12-21(33-23(19)24(27)28-14-29-33)17-7-6-8-18(11-17)31-9-10-32(16(3)34)26(4,5)25(31)35/h6-8,11-15H,9-10H2,1-5H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239723
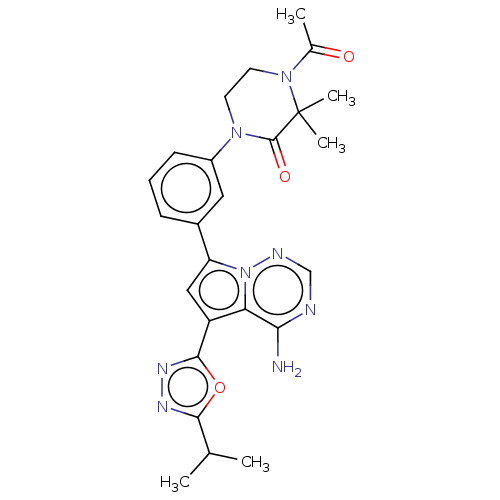
(CHEMBL4074193 | US10214537, Example 478)Show SMILES CC(C)c1nnc(o1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C25H28N8O3/c1-14(2)22-29-30-23(36-22)18-12-19(33-20(18)21(26)27-13-28-33)16-7-6-8-17(11-16)31-9-10-32(15(3)34)25(4,5)24(31)35/h6-8,11-14H,9-10H2,1-5H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239734
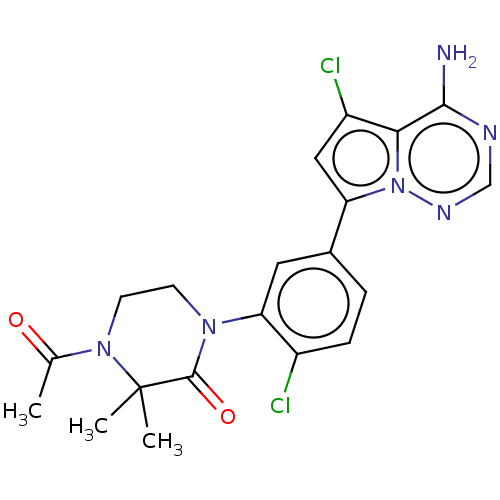
(CHEMBL4097222 | US10214537, Example 628)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1Cl)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H20Cl2N6O2/c1-11(29)27-7-6-26(19(30)20(27,2)3)16-8-12(4-5-13(16)21)15-9-14(22)17-18(23)24-10-25-28(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532540

(CHEMBL4475594)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN Show InChI InChI=1S/C22H22N4O3/c1-2-6-17-19(25-28-20(17)15-7-4-3-5-8-15)22-24-21(26-29-22)16-11-9-14(10-12-16)18(27)13-23/h3-5,7-12,18,27H,2,6,13,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50532540

(CHEMBL4475594)Show SMILES CCCc1c(noc1-c1ccccc1)-c1nc(no1)-c1ccc(cc1)C(O)CN Show InChI InChI=1S/C22H22N4O3/c1-2-6-17-19(25-28-20(17)15-7-4-3-5-8-15)22-24-21(26-29-22)16-11-9-14(10-12-16)18(27)13-23/h3-5,7-12,18,27H,2,6,13,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239716
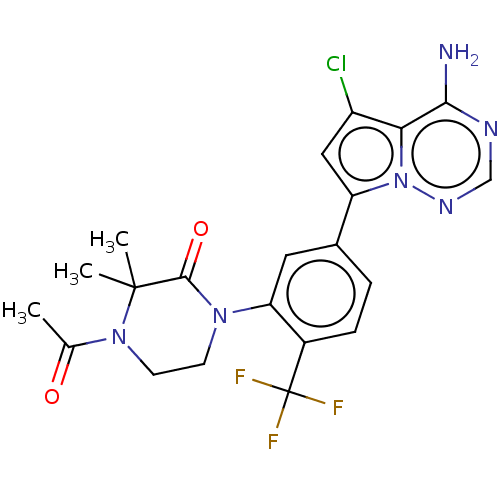
(CHEMBL4078237 | US10214537, Example 587)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C(F)(F)F)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H20ClF3N6O2/c1-11(32)30-7-6-29(19(33)20(30,2)3)16-8-12(4-5-13(16)21(23,24)25)15-9-14(22)17-18(26)27-10-28-31(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate after 3 hrs by ADP-Glo assay |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718

(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data